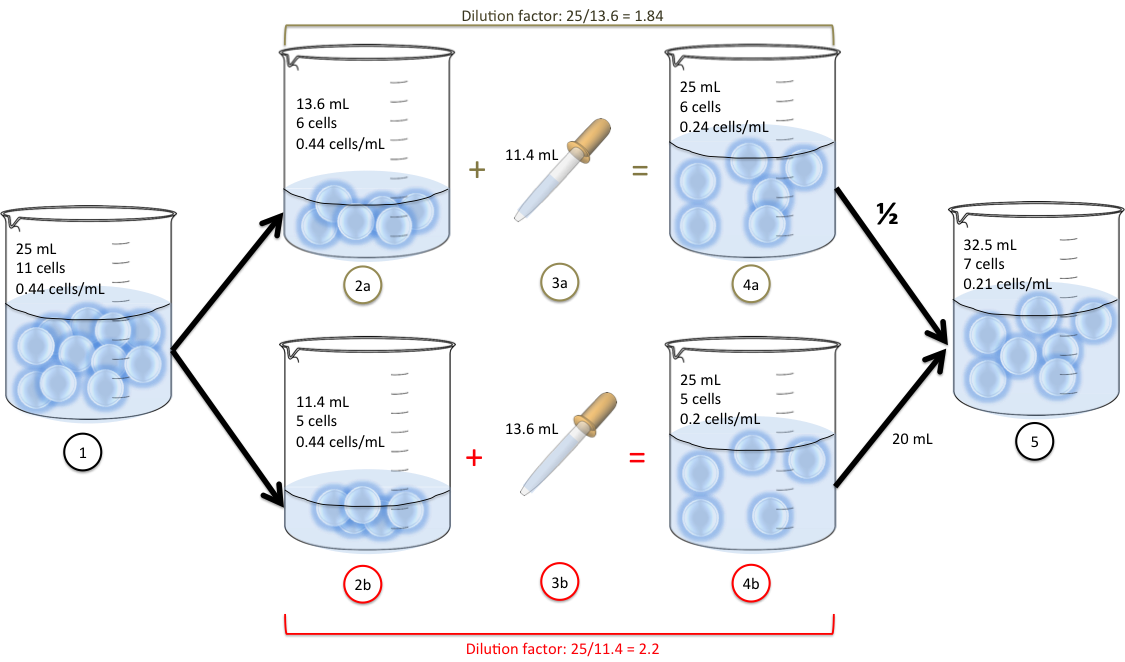
Dilution The Formula . See an example of how to use the formula to. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. M₁v₁ = m₂v₂ where m₁. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes.
from www.hemocytometer.org
learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. M₁v₁ = m₂v₂ where m₁. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. See an example of how to use the formula to. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,.
Using the dilution factor to calculate dilutions • Hemocytometer
Dilution The Formula learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. we can relate the concentrations and volumes before and after a dilution using the following equation: learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. See an example of how to use the formula to. M₁v₁ = m₂v₂ where m₁. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original.
From www.studocu.com
1. Serial Dilution Calculations Dilution Plating Questions Question Dilution The Formula learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. See an example of how to use the formula to. learn how to use the dilution equation m1v1. Dilution The Formula.
From exoubqlmz.blob.core.windows.net
Dilution Equation With at Sharon Firestone blog Dilution The Formula learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn. Dilution The Formula.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution The Formula learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. See an example of how. Dilution The Formula.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution The Formula learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution.. Dilution The Formula.
From chem.libretexts.org
14.7 Solution Dilution Chemistry LibreTexts Dilution The Formula learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. See an example of how to use the formula to. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn how to use the dilution equation m1v1 = m2v2 to calculate. Dilution The Formula.
From www.medicine.mcgill.ca
Serial Dilutions Dilution The Formula learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. See an example of how to use the. Dilution The Formula.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution The Formula M₁v₁ = m₂v₂ where m₁. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume. Dilution The Formula.
From yellowdig263.weebly.com
Serial Dilution Vs Parallel Dilution Dilution The Formula M₁v₁ = m₂v₂ where m₁. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. we can relate the concentrations and volumes before and after a dilution using. Dilution The Formula.
From www.youtube.com
Chem143 Dilution Equation YouTube Dilution The Formula learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. M₁v₁ = m₂v₂ where m₁. See an example of how to use the formula to. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn how to change concentrations of solutions. Dilution The Formula.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution The Formula learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. See an example of how to use the formula to. learn how to calculate the molarity of a diluted solution. Dilution The Formula.
From www.pdfprof.com
how to calculate dilution factor Dilution The Formula See an example of how to use the formula to. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to calculate the molarity of a diluted solution using the dilution. Dilution The Formula.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution The Formula we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. M₁v₁ = m₂v₂ where m₁. learn how to change concentrations of solutions by dilution, a process that involves adding more. Dilution The Formula.
From socratic.org
How can I calculate the dilution factor using concentration? Socratic Dilution The Formula learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. M₁v₁ = m₂v₂ where m₁. See an example of how to use the formula to. we can relate the concentrations and volumes before and after a dilution using the following equation: learn the simple formula of c1v1 = c2v2 to. Dilution The Formula.
From chem2u.blogspot.com
chem2U Dilution method formula Dilution The Formula learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution. Dilution The Formula.
From cfmuvi.jimdo.com
Simple Serial Dilution Calculation cfmuvi Dilution The Formula we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. M₁v₁ = m₂v₂ where m₁. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1. Dilution The Formula.
From dxocmttnm.blob.core.windows.net
Dilution Equation Formulas at Kathleen Milford blog Dilution The Formula M₁v₁ = m₂v₂ where m₁. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. See an example of how to use the formula to. learn how to. Dilution The Formula.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution The Formula See an example of how to use the formula to. we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to use the dilution equation m1v1 = m2v2 to calculate the new. Dilution The Formula.
From mfawriting332.web.fc2.com
How do you calculate dilution? Dilution The Formula See an example of how to use the formula to. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. we can relate the concentrations and volumes. Dilution The Formula.
From www.labpedia.net
Solutions Part 1 Solutions Preparation used in Clinical Laboratory Dilution The Formula learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2.. Dilution The Formula.
From www.youtube.com
Serial Dilution Methods & Calaculations YouTube Dilution The Formula learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or.. Dilution The Formula.
From mfawriting332.web.fc2.com
How do you calculate dilution? Dilution The Formula we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to change concentrations of. Dilution The Formula.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution The Formula learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. See an example of how. Dilution The Formula.
From dxomlaufv.blob.core.windows.net
Dilutions Problems at Cynthia Carroll blog Dilution The Formula we can relate the concentrations and volumes before and after a dilution using the following equation: See an example of how to use the formula to. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. M₁v₁ = m₂v₂ where m₁. learn how to use the dilution equation to calculate the. Dilution The Formula.
From www.slideserve.com
PPT Concentration of Solutions PowerPoint Presentation, free download Dilution The Formula learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. we can. Dilution The Formula.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution The Formula we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn. Dilution The Formula.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilution The Formula M₁v₁ = m₂v₂ where m₁. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. we can relate the concentrations and volumes before and after a dilution using the following equation:. Dilution The Formula.
From www.slideserve.com
PPT Analytical Chemistry PowerPoint Presentation, free download ID Dilution The Formula learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. See an example of how to use the formula to. M₁v₁ = m₂v₂ where m₁. learn how to use the dilution equation. Dilution The Formula.
From sciencestruck.com
Here's How to Calculate Dilution Factor from Given Concentration Dilution The Formula learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and. Dilution The Formula.
From www.hemocytometer.org
Using the dilution factor to calculate dilutions • Hemocytometer Dilution The Formula learn how to use the dilution equation to calculate the final concentration or volume of a diluted solution. See an example of how to use the formula to. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. we can relate the concentrations and volumes before and after a dilution using. Dilution The Formula.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution The Formula learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. learn how to calculate the concentration of a chemical solution using different units, such as percent composition by. Dilution The Formula.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution The Formula learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. See. Dilution The Formula.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution The Formula learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. M₁v₁ = m₂v₂ where m₁. learn how to calculate the concentration of a chemical solution using different units, such as percent composition by mass, volume percent,. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations. Dilution The Formula.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution The Formula learn how to use the dilution equation m1v1 = m2v2 to calculate the new concentration or volume of a solution after adding or. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. See an example of how to use the formula to. learn how to use the dilution equation to. Dilution The Formula.
From www.majordifferences.com
Difference between Dilution and Dilution Factor in Microbiology Dilution The Formula learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. See an example of how to use the formula to. learn how to calculate the molarity of a diluted solution using the dilution equation m1v1 = m2v2. learn the simple formula of c1v1 = c2v2 to dilute solutions. Dilution The Formula.
From exorkoxox.blob.core.windows.net
Dilution Volume Formula at Callie Douglass blog Dilution The Formula we can relate the concentrations and volumes before and after a dilution using the following equation: learn how to change concentrations of solutions by dilution, a process that involves adding more solvent to the original. learn the simple formula of c1v1 = c2v2 to dilute solutions with different concentrations and volumes. M₁v₁ = m₂v₂ where m₁. . Dilution The Formula.