Standard Heat In Combustion . 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation.
from www.grc.nasa.gov
the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines.
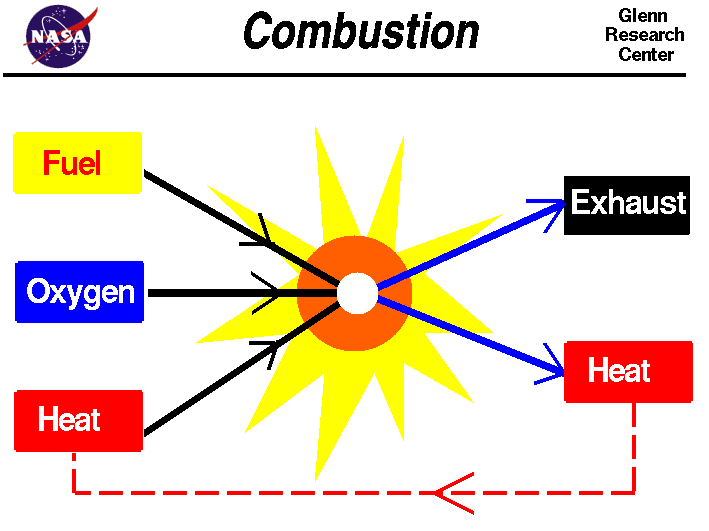
Combustion
Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and.
From spmchemistry.blog.onlinetuition.com.my
Heat of Combustion SPM Chemistry Standard Heat In Combustion 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance. Standard Heat In Combustion.
From www.numerade.com
SOLVED 'The following problems deal with standard enthalpy of Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of. Standard Heat In Combustion.
From www.coursehero.com
[Solved] choose all that apply which have enthalpies of combustion that Standard Heat In Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance. Standard Heat In Combustion.
From www.chegg.com
Solved 9.9. The standard heat of combustion of gaseous Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of. Standard Heat In Combustion.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance,. Standard Heat In Combustion.
From www.coursehero.com
[Solved] Calculate the heat of combustion in kJ 1.0000 mol of ethyl Standard Heat In Combustion learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance. Standard Heat In Combustion.
From maraferstravis.blogspot.com
Standard Enthalpy of Combustion Standard Heat In Combustion the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and. Standard Heat In Combustion.
From www.toppr.com
At 298K , the standard enthalpy of combustion of sucrose is 5737kJ Standard Heat In Combustion the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines.. Standard Heat In Combustion.
From www.researchgate.net
Enthalpy of formation and heats of formation of main combustion Standard Heat In Combustion the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a. Standard Heat In Combustion.
From haipernews.com
How To Calculate Heat Of The Combustion Haiper Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants. Standard Heat In Combustion.
From www.youtube.com
Enthalpy of Formation Reaction & Heat of Combustion, Enthalpy Change Standard Heat In Combustion 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the. Standard Heat In Combustion.
From www.youtube.com
5.1 Standard enthalpy changes of formation and combustion YouTube Standard Heat In Combustion 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of. Standard Heat In Combustion.
From www.coursehero.com
[Solved] Which of the enthalpies of combustion in Table 5.2 table are Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance. Standard Heat In Combustion.
From www.chegg.com
Solved What is the standard heat of combustion of npentane Standard Heat In Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. the heat of combustion is a useful calculation for analyzing the amount of energy. Standard Heat In Combustion.
From www.grc.nasa.gov
Combustion Standard Heat In Combustion learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance,. Standard Heat In Combustion.
From www.chegg.com
Solved Enthalpy of Combustion Alkanes Write a balanced Standard Heat In Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. learn how to calculate the heat of combustion (energy content) of a substance using the standard. Standard Heat In Combustion.
From www.coursehero.com
[Solved] . From the data in the tables, calculate the heat of Standard Heat In Combustion learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a. Standard Heat In Combustion.
From haipernews.com
How To Calculate Heat Of The Combustion Haiper Standard Heat In Combustion the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy. Standard Heat In Combustion.
From www.scribd.com
Module 2 Heat Effects Ii Standard Heat of Reaction PDF Standard Heat In Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84 rows learn about the heat of combustion, the amount of heat released during the combustion. Standard Heat In Combustion.
From spmchemistry.blog.onlinetuition.com.my
Fuel Value SPM Chemistry Standard Heat In Combustion learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard. Standard Heat In Combustion.
From www.youtube.com
The enthalpies of combustion of carbon and carbon monoxide are `390 kJ Standard Heat In Combustion the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance. Standard Heat In Combustion.
From www.slideserve.com
PPT Advanced Thermodynamics Note 3 Heat Effects PowerPoint Standard Heat In Combustion the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and.. Standard Heat In Combustion.
From www.chegg.com
Solved Table 1.13 Heats of combustion of selected fuels at Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a. Standard Heat In Combustion.
From www.slideshare.net
Heat of combustion of Various Alcohols Standard Heat In Combustion the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely. Standard Heat In Combustion.
From svanfitz.blogspot.com
Enthalpy Change Of Combustion PPT ENTHALPY OF FORMATION Combustion Standard Heat In Combustion learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state.. Standard Heat In Combustion.
From www.researchgate.net
(a) The total heat of combustion measured through the experimentations Standard Heat In Combustion the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy. Standard Heat In Combustion.
From www.fatherskit.co
combustion of glucose enthalpy calculate the heat of combustion Standard Heat In Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a. Standard Heat In Combustion.
From maraferstravis.blogspot.com
Standard Enthalpy of Combustion Standard Heat In Combustion the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a. Standard Heat In Combustion.
From ko.wukihow.com
연소열 계산 방법 Standard Heat In Combustion the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance. Standard Heat In Combustion.
From www.slideserve.com
PPT Energy changes PowerPoint Presentation, free download ID3003657 Standard Heat In Combustion learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy of formation. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole.. Standard Heat In Combustion.
From www.engineeringa2z.com
Heat Engine Internal Combustion Engine Engineeringa2z Standard Heat In Combustion the standard enthalpy of combustion (δh°) is the enthalpy change when 1 mole of a substance burns under standard state. the molar heat of combustion \(\left( he \right)\) is the heat released when one mole of a substance is completely burned. 84 rows learn about the heat of combustion, the amount of heat released during the combustion. Standard Heat In Combustion.
From byjus.com
The heat of combustion of CH4(g),graphite and H2(g) are 20 kcal, 40 Standard Heat In Combustion To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. learn how to calculate the heat of combustion (energy content) of a substance using the. Standard Heat In Combustion.
From www.numerade.com
SOLVED The heat of combustion of acetylene, C2H2(g) + O2(g) → CO2(g Standard Heat In Combustion learn the definition and calculation of standard enthalpy of combustion (δh∘c), the heat produced or consumed when 1 mole. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines. the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. . Standard Heat In Combustion.
From www.youtube.com
Molar Heat of Combustion Calculations YouTube Standard Heat In Combustion the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. To calculate the heat of combustion, use hess’s law, which states that the enthalpies of the products and the reactants are the same. learn how to calculate the heat of combustion (energy content) of a substance using the standard enthalpy. Standard Heat In Combustion.
From byjus.com
Calculate standard heat of combustion of ethanol(C2H5OH(I)). Given that Standard Heat In Combustion 84 rows learn about the heat of combustion, the amount of heat released during the combustion of a substance, and. the heat of combustion is a useful calculation for analyzing the amount of energy in a given fuel. standard enthalpy of combustio n (\ (δh_c^\circ\)) is the enthalpy change when 1 mole of a substance burns (combines.. Standard Heat In Combustion.