Mg Nuclear Charge . We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. You will see some examples and get a quick review of the quantum theory behind. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Let’s understand what this statement means. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences.
from www.bartleby.com
Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. You will see some examples and get a quick review of the quantum theory behind. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Let’s understand what this statement means. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules.
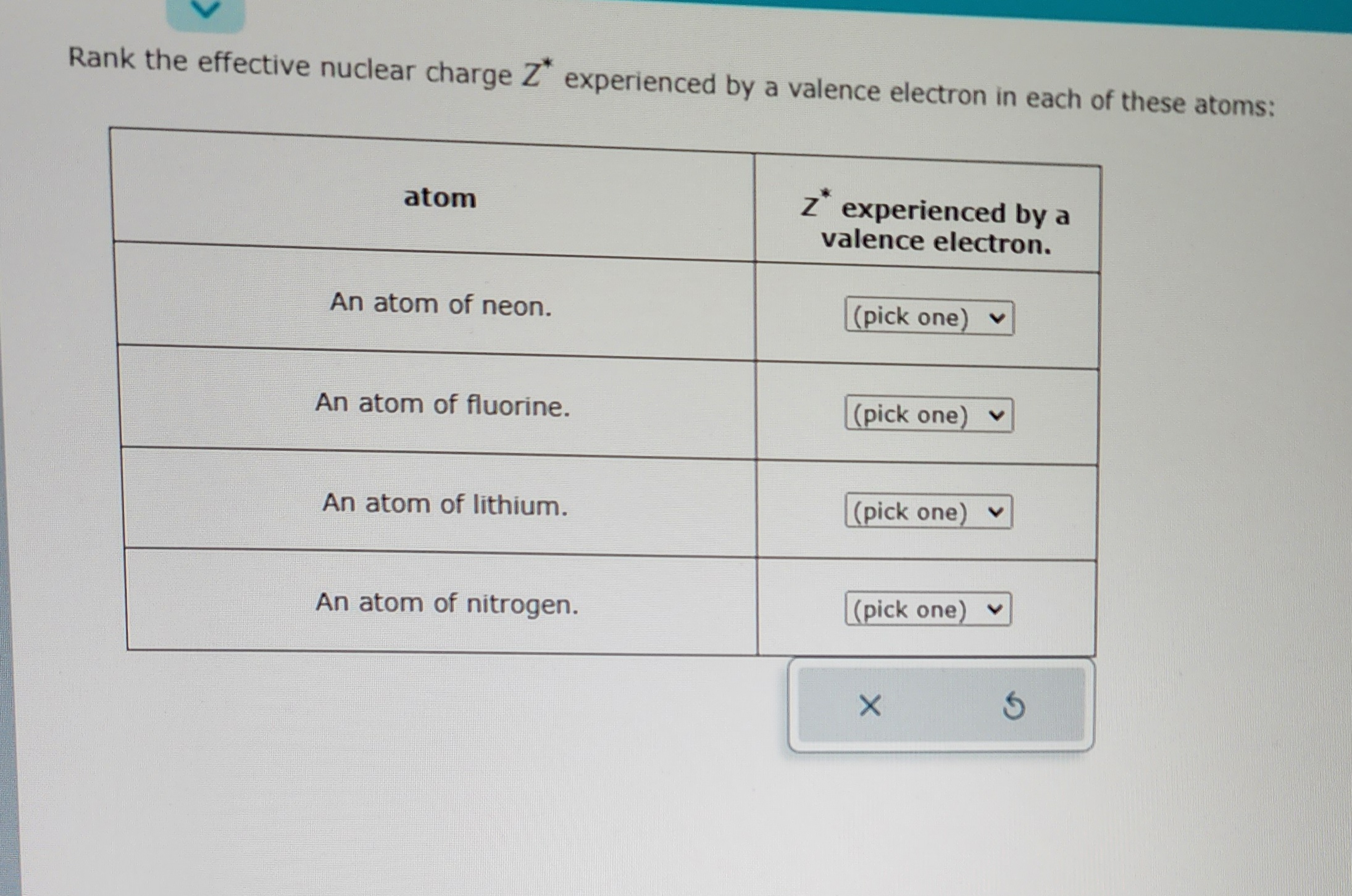
Answered Rank the effective nuclear charge Z*… bartleby
Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. You will see some examples and get a quick review of the quantum theory behind. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Let’s understand what this statement means.
From www.chegg.com
Solved Calculate the effective nuclear charge experienced by Mg Nuclear Charge We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. You will see some examples and get a quick review of the quantum theory behind. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)). Mg Nuclear Charge.
From www.slideserve.com
PPT Periodic Variation in Physical Properties PowerPoint Presentation Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Trends in atomic size result from differences in the. Mg Nuclear Charge.
From www.pearson.com
Which experience a greater effective nuclear charge the valence Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. You will see some examples and get a quick review of the quantum theory behind. We know from basic. Mg Nuclear Charge.
From www.slideserve.com
PPT Chapter 7 Periodic Properties of the Elements PowerPoint Mg Nuclear Charge Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Let’s understand what this statement means.. Mg Nuclear Charge.
From ar.inspiredpencil.com
Effective Nuclear Charge Chart Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is. Mg Nuclear Charge.
From www.chegg.com
Solved Explain why the atomic radius of Mg is smaller than Mg Nuclear Charge Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. You will see some examples and get a quick. Mg Nuclear Charge.
From www.nuclear-power.com
Magnesium Atomic Number Atomic Mass Density of Magnesium Mg Nuclear Charge An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Trends in atomic size result from differences in. Mg Nuclear Charge.
From www.thesciencehive.co.uk
Atomic Structure (GCSE) — the science hive Mg Nuclear Charge Let’s understand what this statement means. You will see some examples and get a quick review of the quantum theory behind. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of. Mg Nuclear Charge.
From hillruslaideemin.blogspot.com
Hill Ruslaideemin Mg Nuclear Charge You will see some examples and get a quick review of the quantum theory behind. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Here you will learn what the effective nuclear charge. Mg Nuclear Charge.
From cabinet.matttroy.net
Properties Of Magnesium On The Periodic Table Matttroy Mg Nuclear Charge An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Here you will learn. Mg Nuclear Charge.
From general.chemistrysteps.com
Effective Nuclear Charge Chemistry Steps Mg Nuclear Charge You will see some examples and get a quick review of the quantum theory behind. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Here you will learn what the effective nuclear charge is and how to. Mg Nuclear Charge.
From facts.net
11 Captivating Facts About Effective Nuclear Charge Mg Nuclear Charge Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. You will see some examples and get a quick review of the quantum theory behind. Let’s understand. Mg Nuclear Charge.
From sciencenotes.org
Nuclear Symbol Notation Mg Nuclear Charge You will see some examples and get a quick review of the quantum theory behind. Let’s understand what this statement means. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. An effective nuclear. Mg Nuclear Charge.
From www.numerade.com
SOLVED Write the full electronic configurations of both Mg and Mg2 Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Let’s understand what this statement means. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. An effective nuclear charge. Mg Nuclear Charge.
From enginedatanichered.z21.web.core.windows.net
Atomic Diagram Of Magnesium Mg Nuclear Charge You will see some examples and get a quick review of the quantum theory behind. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. We know from basic physics that opposite electrical charges attract, and if. Mg Nuclear Charge.
From wiredbbagley.z13.web.core.windows.net
Magnesium Electron Dot Diagram Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Let’s understand what this statement means. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements.. Mg Nuclear Charge.
From www.numerade.com
SOLVEDArrange the following atoms in order of increasing effective Mg Nuclear Charge Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. You will see some examples and get a quick review of the quantum theory behind. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to. Mg Nuclear Charge.
From www.youtube.com
Effective Nuclear Charge Chemistry Tutorial YouTube Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an. Mg Nuclear Charge.
From chem.libretexts.org
7.5 Atomic Properties and Periodic Trends Chemistry LibreTexts Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force. Mg Nuclear Charge.
From mmaawarrhitam.blogspot.com
As Zeff Increases The Electron Experiencing That Zeff Will / Periodic Mg Nuclear Charge An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Let’s understand what this statement means. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. We know from. Mg Nuclear Charge.
From www.breakingatom.com
Nuclear Charge Mg Nuclear Charge We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. You will see some examples and get a quick review of the quantum theory behind. Here you will learn what the effective nuclear charge is and how to. Mg Nuclear Charge.
From animalia-life.club
Isotope Symbol Examples Mg Nuclear Charge We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Let’s understand what this statement means. An effective nuclear charge. Mg Nuclear Charge.
From www.youtube.com
How To Use Slater's Rule to Estimate The Effective Nuclear Charge YouTube Mg Nuclear Charge Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Let’s understand what this statement means. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it. Mg Nuclear Charge.
From www.studocu.com
First half of worksheet 4 1. Since E p2 and Mg 24 has a greater Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. You will see some examples and get a quick review of the quantum theory behind. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an. Mg Nuclear Charge.
From www.slideserve.com
PPT Atomic Electron Configurations and Chemical Periodicity Mg Nuclear Charge Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. You will see some examples and. Mg Nuclear Charge.
From periodictableguide.com
Magnesium (Mg) Periodic Table (Element Information & More) Mg Nuclear Charge Let’s understand what this statement means. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. An effective nuclear charge calculator is a tool used. Mg Nuclear Charge.
From www.numerade.com
SOLVED(a) Calculate the effective nuclear charge, Z^*, on a 2 p Mg Nuclear Charge An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Let’s understand what this statement means. You will see some examples and get a quick review of. Mg Nuclear Charge.
From www.dreamstime.com
Magnesium Mg Chemical Element. Magnesium Sign with Atomic Number Mg Nuclear Charge Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. Let’s understand what this statement means. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. You will see some examples and get a quick review of. Mg Nuclear Charge.
From www.bartleby.com
Answered Rank the effective nuclear charge Z*… bartleby Mg Nuclear Charge You will see some examples and get a quick review of the quantum theory behind. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)). Mg Nuclear Charge.
From sites.google.com
Atomic Structure Protons, Neutrons and Electrons Mrs. Sanborn's Site Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. You will see some examples and get a quick review of the quantum theory behind. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an. Mg Nuclear Charge.
From www.numerade.com
SOLVEDArrange the atoms according to decreasing effective nuclear Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. You will see some examples and get a quick review of the quantum theory behind. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. We know from basic physics that opposite. Mg Nuclear Charge.
From www.britannica.com
Magnesium Description, Properties, & Compounds Britannica Mg Nuclear Charge Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. Let’s understand what this statement means. Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. We know from. Mg Nuclear Charge.
From sciencenotes.org
Atomic Radius and Ionic Radius Mg Nuclear Charge We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Let’s understand what this statement means. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the. Mg Nuclear Charge.
From www.slideserve.com
PPT Nuclear model of atom PowerPoint Presentation, free download ID Mg Nuclear Charge Here you will learn what the effective nuclear charge is and how to calculate it using slater's rules. We know from basic physics that opposite electrical charges attract, and if we consider the hydrogen atom, it is fairly straightforward to understand that there is an attractive force between the positively. Trends in atomic size result from differences in the effective. Mg Nuclear Charge.
From valenceelectrons.com
How to Write the Electron Configuration for Magnesium (Mg)? Mg Nuclear Charge Effective nuclear charge (z eff) is the nuclear charge an electron actually experiences. Trends in atomic size result from differences in the effective nuclear charges (\(z_{eff}\)) experienced by electrons in the outermost orbitals of the elements. An effective nuclear charge calculator is a tool used in chemistry to determine the actual electrostatic attraction between an atom’s. You will see some. Mg Nuclear Charge.