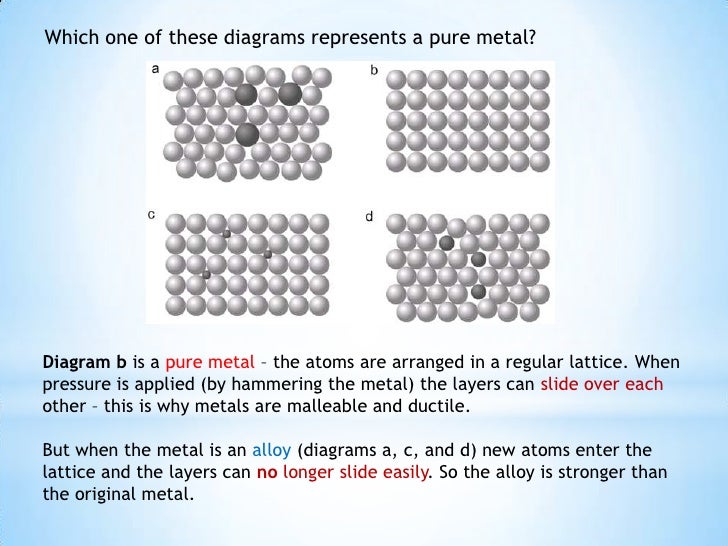
Why Are Metals Malleable Igcse . good conductors of heat and electricity because of free electrons that can move throughout the metal. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. Compare the general physical properties of metals and non. metallic bonds are very strong so the giant metallic structure is strongly held together. This is because they consist of layers of ions that. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in.
from www.slideshare.net
metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. This is because they consist of layers of ions that. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Compare the general physical properties of metals and non. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metallic bonds are very strong so the giant metallic structure is strongly held together. good conductors of heat and electricity because of free electrons that can move throughout the metal.
Metals
Why Are Metals Malleable Igcse This is because they consist of layers of ions that. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. metallic bonds are very strong so the giant metallic structure is strongly held together. Compare the general physical properties of metals and non. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. good conductors of heat and electricity because of free electrons that can move throughout the metal. This is because they consist of layers of ions that.
From chemnotcheem.com
Metallic bonding & giant metallic structure O Level Chemistry Notes Why Are Metals Malleable Igcse Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. good conductors of heat and electricity because of free electrons that can move throughout the metal. This is because they consist of layers of ions that. Compare the general physical properties of metals and non. metallic bonds are very. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Metals PowerPoint Presentation ID1462875 Why Are Metals Malleable Igcse Compare the general physical properties of metals and non. This is because they consist of layers of ions that. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. good. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Worksheet Chapter 12 Homework Key PowerPoint Presentation, free Why Are Metals Malleable Igcse Compare the general physical properties of metals and non. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. good conductors of heat and electricity because of free. Why Are Metals Malleable Igcse.
From www.studypool.com
SOLUTION Metals and extraction of metals cie o level igcse gcse Why Are Metals Malleable Igcse most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. good conductors of heat and electricity because of free electrons that can move throughout the metal. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. revision notes on. Why Are Metals Malleable Igcse.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.54C Explain Typical Physical Properties of Why Are Metals Malleable Igcse revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metallic bonds are very strong so the giant metallic structure is strongly held together. This is because they consist of layers of ions that. Compare the general physical properties of metals and non. Their malleable and ductile. Why Are Metals Malleable Igcse.
From bitgoldbitcoin1126.blogspot.com
Why Is Gold Malleable 7 Reasons Why Gold Is So Valuable to Us The Why Are Metals Malleable Igcse metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. metallic bonds are very strong so the giant metallic structure is strongly held together. This is because they consist. Why Are Metals Malleable Igcse.
From slideplayer.com
Metals, Nonmetals and Metalloids ppt download Why Are Metals Malleable Igcse most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. . Why Are Metals Malleable Igcse.
From www.youtube.com
4.5 Why are Metals Malleable and Good Conductors? YouTube Why Are Metals Malleable Igcse good conductors of heat and electricity because of free electrons that can move throughout the metal. This is because they consist of layers of ions that. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. revision notes on properties of metals for the cie igcse chemistry syllabus,. Why Are Metals Malleable Igcse.
From slideplayer.com
Metallic Bonds. ppt download Why Are Metals Malleable Igcse metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. This is because they consist of layers of ions that. good conductors of heat and electricity because of free electrons that can move throughout the metal. revision notes on properties of metals for the cie igcse chemistry syllabus,. Why Are Metals Malleable Igcse.
From www.hanlin.com
Edexcel IGCSE Chemistry 复习笔记 2.5.3 Using Metals翰林国际教育 Why Are Metals Malleable Igcse Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metallic bonds are very strong so the giant metallic structure is strongly held together. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Compare the general physical properties of. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Chapter 15 Ionic Bonding and Ionic Compounds PowerPoint Why Are Metals Malleable Igcse Compare the general physical properties of metals and non. metallic bonds are very strong so the giant metallic structure is strongly held together. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry. Why Are Metals Malleable Igcse.
From www.prestigewroughtiron.com.au
Why Are Metals Malleable? The Sciene Behind It Why Are Metals Malleable Igcse Compare the general physical properties of metals and non. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. metallic bonds are very strong so the giant metallic structure. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Chapter 7 “Metallic Bonding” PowerPoint Presentation, free Why Are Metals Malleable Igcse This is because they consist of layers of ions that. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Compare the general physical properties of metals and non. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. Their. Why Are Metals Malleable Igcse.
From www.thesciencehive.co.uk
Metallic Bonding (GCSE) — the science sauce Why Are Metals Malleable Igcse Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. This is because they consist of layers of ions that. Compare the general physical properties of metals and non. good. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2696346 Why Are Metals Malleable Igcse metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metallic bonds are very strong so the giant metallic structure is strongly held together. most metals are malleable because. Why Are Metals Malleable Igcse.
From www.youtube.com
Thermal of Metals Cambridge IGCSE O level Chemistry 0620 Why Are Metals Malleable Igcse good conductors of heat and electricity because of free electrons that can move throughout the metal. This is because they consist of layers of ions that. metallic bonds are very strong so the giant metallic structure is strongly held together. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial. Why Are Metals Malleable Igcse.
From www.slideshare.net
Metallic Bonding Why Are Metals Malleable Igcse metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. Compare the general physical properties of metals and non. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. This is because they consist of layers of ions that. Their. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Metallic Bonding PowerPoint Presentation, free download ID2041377 Why Are Metals Malleable Igcse Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. . Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT 4.4 Metallic bonding PowerPoint Presentation ID400526 Why Are Metals Malleable Igcse most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. This is because they consist of layers of ions that. Their malleable and ductile nature allows them to be shaped. Why Are Metals Malleable Igcse.
From www.nagwa.com
Question Video Identifying Metal Properties Responsible for Ductility Why Are Metals Malleable Igcse Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. Compare the general physical properties of metals and non. revision notes on properties of metals for the cie igcse chemistry. Why Are Metals Malleable Igcse.
From quizguarantors.z4.web.core.windows.net
What Are Malleable Elements Why Are Metals Malleable Igcse metallic bonds are very strong so the giant metallic structure is strongly held together. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. Compare the general physical properties of metals and non. Their malleable and ductile nature allows them to be shaped into a variety of. Why Are Metals Malleable Igcse.
From www.slideshare.net
Metals Why Are Metals Malleable Igcse revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metallic bonds are very strong so the giant metallic structure is strongly held together. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. Their malleable and. Why Are Metals Malleable Igcse.
From quizguarantors.z4.web.core.windows.net
What Metals Are Malleable Why Are Metals Malleable Igcse good conductors of heat and electricity because of free electrons that can move throughout the metal. Compare the general physical properties of metals and non. This is because they consist of layers of ions that. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metallic. Why Are Metals Malleable Igcse.
From www.studypool.com
SOLUTION Metals and extraction of metals cie o level igcse gcse Why Are Metals Malleable Igcse metallic bonds are very strong so the giant metallic structure is strongly held together. good conductors of heat and electricity because of free electrons that can move throughout the metal. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Their malleable and ductile nature allows them to. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID1128267 Why Are Metals Malleable Igcse metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. good conductors of heat and electricity because of free electrons that can move throughout the metal. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. most metals are. Why Are Metals Malleable Igcse.
From dotandlinelearning.com
Chapter 9 Mastering Metal Science A Comprehensive Guide to IGCSE Why Are Metals Malleable Igcse Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. good conductors of heat and electricity because of free electrons that can move throughout the metal. This is because they. Why Are Metals Malleable Igcse.
From chayagrocervantes.blogspot.com
Why Are Metals Malleable ChayagroCervantes Why Are Metals Malleable Igcse This is because they consist of layers of ions that. Compare the general physical properties of metals and non. good conductors of heat and electricity because of free electrons that can move throughout the metal. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. revision notes on. Why Are Metals Malleable Igcse.
From byjus.com
Why are metals malleable, ductile, sonorus, lustrous and why are group Why Are Metals Malleable Igcse metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. good conductors of heat and electricity because of free electrons that can move throughout the metal. Compare the general. Why Are Metals Malleable Igcse.
From www.youtube.com
Uses of metals IGCSE Chemistry YouTube Why Are Metals Malleable Igcse This is because they consist of layers of ions that. most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metallic bonds are very strong so the giant metallic structure. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Metals PowerPoint Presentation, free download ID3827489 Why Are Metals Malleable Igcse Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my. Why Are Metals Malleable Igcse.
From www.science-revision.co.uk
Metallic bonding Why Are Metals Malleable Igcse most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my. Why Are Metals Malleable Igcse.
From www.studypool.com
SOLUTION Metals gcse igcse chemistry 0620 notes Studypool Why Are Metals Malleable Igcse most metals are malleable because the atoms can roll over each other and retain the structure of the crystal. This is because they consist of layers of ions that. revision notes on properties of metals for the cie igcse chemistry syllabus, written by the chemistry experts at save my exams. metals as well as their alloys can. Why Are Metals Malleable Igcse.
From www.pinterest.com
Malleable Metal of the periodic table. Periodic table, Metal Why Are Metals Malleable Igcse metallic bonds are very strong so the giant metallic structure is strongly held together. good conductors of heat and electricity because of free electrons that can move throughout the metal. This is because they consist of layers of ions that. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial. Why Are Metals Malleable Igcse.
From byjus.com
Why are metals malleable, ductile, sonorus, lustrous and why are group Why Are Metals Malleable Igcse metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. This is because they consist of layers of ions that. Their malleable and ductile nature allows them to be shaped into a variety of forms without breaking, crucial in. most metals are malleable because the atoms can roll over. Why Are Metals Malleable Igcse.
From www.slideserve.com
PPT Objectives PowerPoint Presentation, free download ID5858440 Why Are Metals Malleable Igcse metallic bonds are very strong so the giant metallic structure is strongly held together. good conductors of heat and electricity because of free electrons that can move throughout the metal. metals as well as their alloys can conduct electricity due to the presence of delocalised electrons in their structure. most metals are malleable because the atoms. Why Are Metals Malleable Igcse.