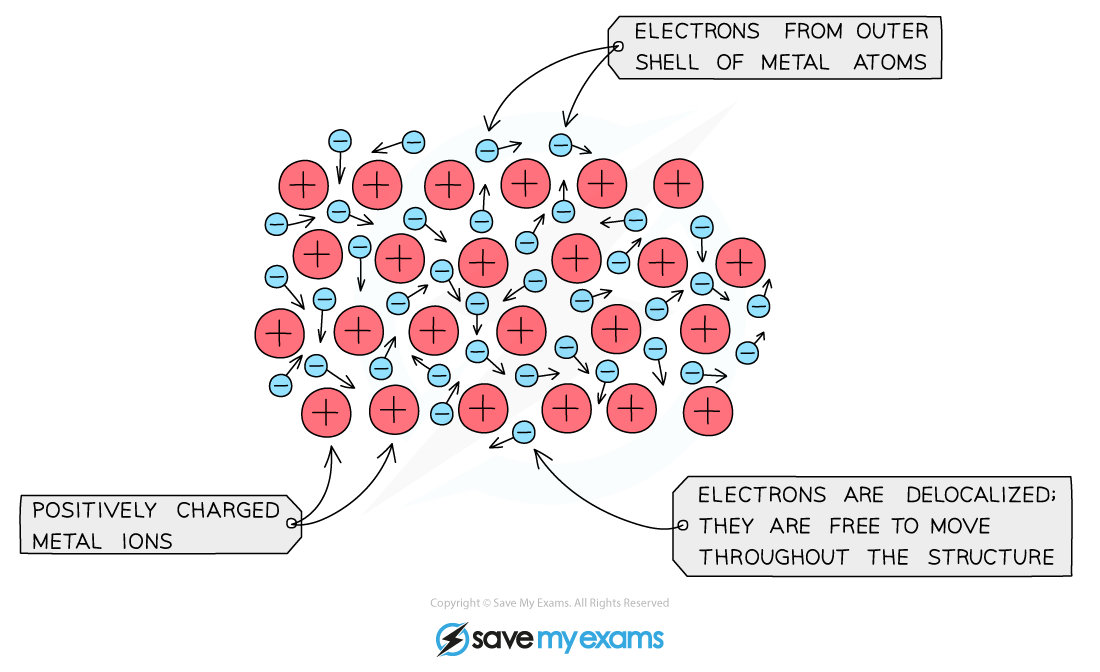
Copper Delocalised Electrons . But it has 1 s electron in the last shell and 10 d electrons. The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. Electrons can move throughout the metal. Instead, they form a “sea” of freely moving electrons throughout the. Metals conduct electricity because they have delocalised electrons. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. As an ion, copper can give off 1, 2, 3 or 4 electrons. So as a metal, how. [higher tier only] the greater the number of outer electrons that the metal has, the higher. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. These carry electrical charge through the metal. This type of bonding occurs in metals and metal alloys, which are.
from www.linstitute.net
But it has 1 s electron in the last shell and 10 d electrons. This type of bonding occurs in metals and metal alloys, which are. The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. [higher tier only] the greater the number of outer electrons that the metal has, the higher. Instead, they form a “sea” of freely moving electrons throughout the. Electrons can move throughout the metal. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. So as a metal, how. Metals conduct electricity because they have delocalised electrons.
Edexcel IGCSE Chemistry 复习笔记 1.8.1 Metallic Bonding翰林国际教育
Copper Delocalised Electrons [higher tier only] the greater the number of outer electrons that the metal has, the higher. The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. As an ion, copper can give off 1, 2, 3 or 4 electrons. These carry electrical charge through the metal. This type of bonding occurs in metals and metal alloys, which are. Instead, they form a “sea” of freely moving electrons throughout the. But it has 1 s electron in the last shell and 10 d electrons. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. Metals conduct electricity because they have delocalised electrons. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. So as a metal, how. [higher tier only] the greater the number of outer electrons that the metal has, the higher. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. Electrons can move throughout the metal.
From soyungringo.blogspot.com
metallic bonding occurs between atoms of best tricktaking card games Copper Delocalised Electrons This type of bonding occurs in metals and metal alloys, which are. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge. Copper Delocalised Electrons.
From valenceelectrons.com
Electron Configuration for Copper (Cu, Cu+, Cu2+) Copper Delocalised Electrons The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. As an ion, copper can give off 1, 2, 3 or 4 electrons. Instead, they form a “sea” of freely moving electrons throughout the. But it has. Copper Delocalised Electrons.
From rex-blogcrosby.blogspot.com
Which Electrons From the Metal Make Up the Delocalized Electrons Copper Delocalised Electrons For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. Instead, they form a “sea” of freely moving electrons throughout the. The following example. Copper Delocalised Electrons.
From www.researchgate.net
(A) Illustration showing the electron delocalization of three reduced Copper Delocalised Electrons Electrons can move throughout the metal. So as a metal, how. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. [higher tier only] the greater the number of outer electrons that the metal has, the higher. Copper atoms’ outermost. Copper Delocalised Electrons.
From www.slideserve.com
PPT METALS PowerPoint Presentation, free download ID3683035 Copper Delocalised Electrons For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. These carry electrical charge through the metal. [higher tier only] the greater the number of outer electrons that the metal has, the higher. So as a metal, how. As an ion, copper can. Copper Delocalised Electrons.
From stock.adobe.com
Metallic bond Metallic bonding is the electrostatic attractive force Copper Delocalised Electrons The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. So as a metal, how. This type of bonding occurs in metals and metal alloys, which are. As an ion, copper can give off 1, 2, 3 or 4 electrons. Metals conduct electricity because they have delocalised electrons. The following example illustrates. Copper Delocalised Electrons.
From www.researchgate.net
11. Coupling of a metal and delocalized ligand π Copper Delocalised Electrons These carry electrical charge through the metal. This type of bonding occurs in metals and metal alloys, which are. The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. Instead, they form a “sea” of freely moving electrons throughout the. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind. Copper Delocalised Electrons.
From www.chemistrystudent.com
Metallic Bonding (ALevel) ChemistryStudent Copper Delocalised Electrons Instead, they form a “sea” of freely moving electrons throughout the. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. These carry electrical charge through the metal. Electrons can move throughout the metal. So as a metal, how. This. Copper Delocalised Electrons.
From www.slideserve.com
PPT Delocalized Electrons Resonance PowerPoint Presentation, free Copper Delocalised Electrons The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. As an ion, copper can give off 1, 2, 3 or 4 electrons. This type of bonding occurs in metals and metal alloys, which are. Instead, they form a “sea” of freely moving electrons throughout the. [higher tier only] the greater the. Copper Delocalised Electrons.
From eleobo.com
How Electricity Flows Through Copper Wires eleobo Copper Delocalised Electrons Instead, they form a “sea” of freely moving electrons throughout the. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. But it has 1 s electron in the last shell and 10 d electrons. Metals conduct electricity because they have delocalised electrons.. Copper Delocalised Electrons.
From www.youtube.com
14.1 Molecules and ions with delocalised pi electrons (HL) YouTube Copper Delocalised Electrons The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. Electrons can move throughout the metal. So as a metal, how. But it has 1 s electron in the last shell and 10 d electrons. These carry. Copper Delocalised Electrons.
From www.mooramo.com
Ions of Transition Elements Mooramo Copper Delocalised Electrons [higher tier only] the greater the number of outer electrons that the metal has, the higher. This type of bonding occurs in metals and metal alloys, which are. But it has 1 s electron in the last shell and 10 d electrons. Metals conduct electricity because they have delocalised electrons. A strong metallic bond will be the result of more. Copper Delocalised Electrons.
From valenceelectrons.com
How Many Valence Electrons Does Copper (Cu) Have? Copper Delocalised Electrons The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. As an ion, copper can give off 1, 2, 3 or 4 electrons. These carry electrical charge through the metal. But it has 1 s electron in the last shell. Copper Delocalised Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper II Easy & Quick YouTube Copper Delocalised Electrons These carry electrical charge through the metal. The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. [higher tier only] the greater. Copper Delocalised Electrons.
From www.coursehero.com
[Solved] 15. Fill in the electron configuration diagram for the copper Copper Delocalised Electrons This type of bonding occurs in metals and metal alloys, which are. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. Electrons can move throughout the metal. [higher tier only] the greater the number of outer electrons that the metal has, the. Copper Delocalised Electrons.
From www.shutterstock.com
691 Metallic Bond Chemistry Images, Stock Photos & Vectors Shutterstock Copper Delocalised Electrons As an ion, copper can give off 1, 2, 3 or 4 electrons. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. These carry electrical charge through the metal. Metals conduct electricity because they have delocalised electrons. So as a metal, how. Instead, they form a “sea” of freely moving electrons throughout the. Electrons. Copper Delocalised Electrons.
From online-learning-college.com
Metallic bonding What is it? Properties of metallic structures Copper Delocalised Electrons As an ion, copper can give off 1, 2, 3 or 4 electrons. So as a metal, how. These carry electrical charge through the metal. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. The following example illustrates how a lone pair of electrons from carbon can be moved to. Copper Delocalised Electrons.
From www.alamy.com
Copper (Cu). Diagram of the nuclear composition, electron configuration Copper Delocalised Electrons [higher tier only] the greater the number of outer electrons that the metal has, the higher. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. So as a metal, how. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle. Copper Delocalised Electrons.
From www.chemistrystudent.com
Metallic Bonding (ALevel) ChemistryStudent Copper Delocalised Electrons For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. Metals conduct electricity because they have delocalised electrons. But it has 1 s electron in the last shell and 10 d electrons. These carry electrical charge through the metal. Instead, they form a. Copper Delocalised Electrons.
From evulpo.com
evulpo Giant covalent and metallic structures Copper Delocalised Electrons As an ion, copper can give off 1, 2, 3 or 4 electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. This. Copper Delocalised Electrons.
From www.slideserve.com
PPT Chemistry 100 Chapter 9 PowerPoint Presentation, free download Copper Delocalised Electrons The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. As an ion, copper can give off 1, 2, 3 or 4 electrons. Electrons can move throughout the metal. These carry electrical charge through the metal. Instead, they form a. Copper Delocalised Electrons.
From periodictable.me
How To Find A Electron Configuration For Copper Dynamic Periodic Copper Delocalised Electrons The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. So as a metal, how. This type of bonding occurs in. Copper Delocalised Electrons.
From www.flickr.com
Metal structure delocalised electrons Illustration used … Flickr Copper Delocalised Electrons Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. Metals conduct electricity because they have delocalised electrons. [higher tier only] the greater the number of outer electrons that the metal has, the higher. But it. Copper Delocalised Electrons.
From elchoroukhost.net
Copper Periodic Table Protons Neutrons And Electrons Elcho Table Copper Delocalised Electrons A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized over all three copper atoms. Electrons can move throughout the metal. This type of bonding occurs in metals and. Copper Delocalised Electrons.
From slideplayer.com
Basic Concepts of Chemical Bonding ppt download Copper Delocalised Electrons The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. Metals conduct electricity because they have delocalised electrons. So as a metal, how. These carry electrical charge through the metal. For cu 3 + cluster, the results found that a pair of electrons is located inside of the copper triangle being delocalized. Copper Delocalised Electrons.
From www.thoughtco.com
Atom Diagrams Electron Configurations of the Elements Copper Delocalised Electrons As an ion, copper can give off 1, 2, 3 or 4 electrons. [higher tier only] the greater the number of outer electrons that the metal has, the higher. Electrons can move throughout the metal. Metals conduct electricity because they have delocalised electrons. But it has 1 s electron in the last shell and 10 d electrons. This type of. Copper Delocalised Electrons.
From www.pinterest.com
Delocalized electron definition and examples. Chemistry lessons Copper Delocalised Electrons Instead, they form a “sea” of freely moving electrons throughout the. [higher tier only] the greater the number of outer electrons that the metal has, the higher. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on. So as a metal, how. But it has 1 s electron in the last. Copper Delocalised Electrons.
From www.linstitute.net
Edexcel IGCSE Chemistry 复习笔记 1.8.1 Metallic Bonding翰林国际教育 Copper Delocalised Electrons [higher tier only] the greater the number of outer electrons that the metal has, the higher. These carry electrical charge through the metal. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. The metallic bond is the strong force. Copper Delocalised Electrons.
From www.slideserve.com
PPT Delocalized Electrons Resonance PowerPoint Presentation, free Copper Delocalised Electrons As an ion, copper can give off 1, 2, 3 or 4 electrons. Instead, they form a “sea” of freely moving electrons throughout the. Electrons can move throughout the metal. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons.. Copper Delocalised Electrons.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 1.54C Explain Typical Physical Properties of Copper Delocalised Electrons Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. Metals conduct electricity because they have delocalised electrons. Instead, they form a “sea” of freely moving electrons throughout the. These carry electrical charge through the metal. So as a metal, how. But it has 1 s electron in the last shell and 10 d electrons.. Copper Delocalised Electrons.
From ninakruwlynch.blogspot.com
Which Electrons From the Metal Make Up the Delocalized Electrons Copper Delocalised Electrons The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. [higher tier only] the greater the number of outer electrons that the metal has, the higher. This type of bonding occurs in metals and metal alloys, which are. Metals conduct. Copper Delocalised Electrons.
From www.thesciencehive.co.uk
Periodicity* — the science sauce Copper Delocalised Electrons But it has 1 s electron in the last shell and 10 d electrons. [higher tier only] the greater the number of outer electrons that the metal has, the higher. This type of bonding occurs in metals and metal alloys, which are. These carry electrical charge through the metal. Metals conduct electricity because they have delocalised electrons. The metallic bond. Copper Delocalised Electrons.
From stock.adobe.com
Periodic Table of the Elements, Shell Structure of Copper Cu Copper Delocalised Electrons But it has 1 s electron in the last shell and 10 d electrons. [higher tier only] the greater the number of outer electrons that the metal has, the higher. Instead, they form a “sea” of freely moving electrons throughout the. Copper atoms’ outermost electrons, known as valence electrons, do not tightly bind to individual atoms. Metals conduct electricity because. Copper Delocalised Electrons.
From studylib.net
Delocalization of Electrons Copper Delocalised Electrons The metallic bond is the strong force of attraction between the positive metal ions and the delocalised electrons. But it has 1 s electron in the last shell and 10 d electrons. Instead, they form a “sea” of freely moving electrons throughout the. [higher tier only] the greater the number of outer electrons that the metal has, the higher. For. Copper Delocalised Electrons.
From www.youtube.com
How to Find the Valence Electrons for Copper (Cu) YouTube Copper Delocalised Electrons Electrons can move throughout the metal. This type of bonding occurs in metals and metal alloys, which are. The following example illustrates how a lone pair of electrons from carbon can be moved to make a new \(\pi\) bond to an adjacent carbon, and how the \(\pi\) electrons. The metallic bond is the strong force of attraction between the positive. Copper Delocalised Electrons.