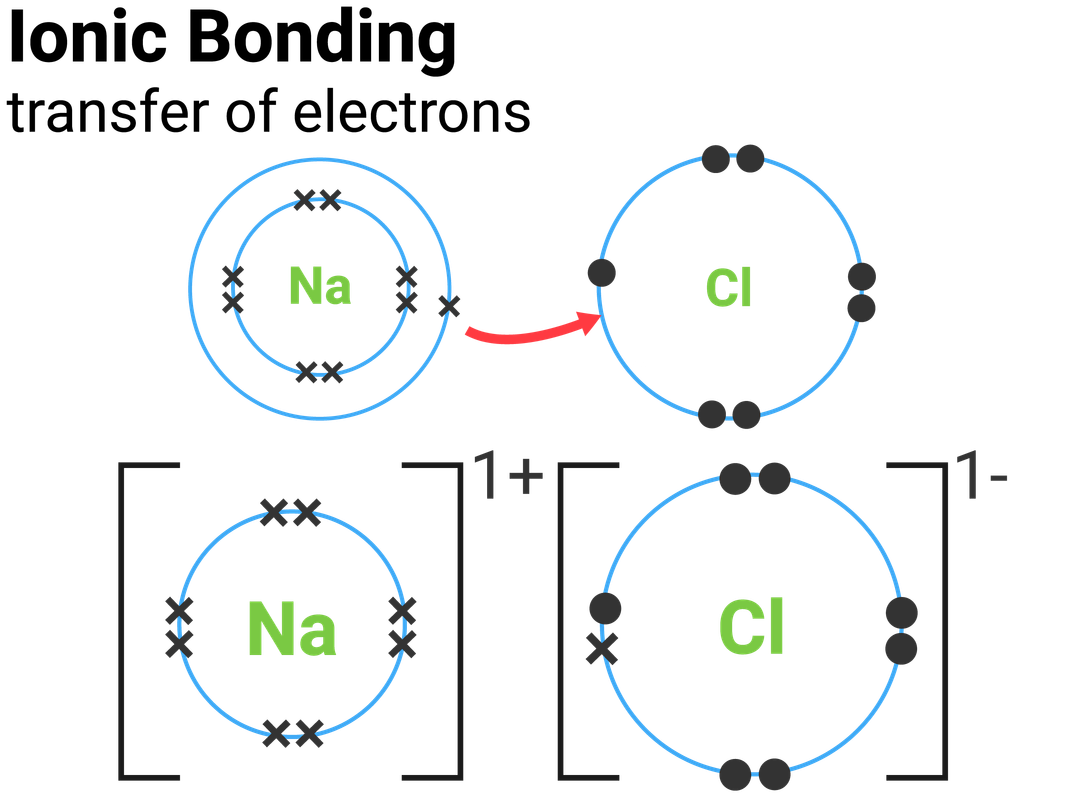
Chlorine And Magnesium Ionic Compound . Therefore, the proper formula for this ionic compound is mgo. Mg + cl = mgcl2 is a synthesis reaction where one. When a metal is combined with one or more nonmetals, the. The attraction between the oppositely charged ions forms the ionic bond between. Two chlorine atoms will each gain one electron from the magnesium atom. Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Magnesium + chlorine = magnesium chloride. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. The periodic table can help us recognize many of the compounds that are ionic: Now consider the ionic compound formed by magnesium and chlorine.
from revisechemistry.uk
The periodic table can help us recognize many of the compounds that are ionic: Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. When a metal is combined with one or more nonmetals, the. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Two chlorine atoms will each gain one electron from the magnesium atom. The attraction between the oppositely charged ions forms the ionic bond between. Therefore, the proper formula for this ionic compound is mgo. Magnesium + chlorine = magnesium chloride. Mg + cl = mgcl2 is a synthesis reaction where one.
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk
Chlorine And Magnesium Ionic Compound Now consider the ionic compound formed by magnesium and chlorine. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. The periodic table can help us recognize many of the compounds that are ionic: Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Therefore, the proper formula for this ionic compound is mgo. Two chlorine atoms will each gain one electron from the magnesium atom. When a metal is combined with one or more nonmetals, the. Now consider the ionic compound formed by magnesium and chlorine. The attraction between the oppositely charged ions forms the ionic bond between. Magnesium + chlorine = magnesium chloride. Mg + cl = mgcl2 is a synthesis reaction where one.
From www.slideshare.net
2012 topic 4.1 bonding ionic Chlorine And Magnesium Ionic Compound When a metal is combined with one or more nonmetals, the. Therefore, the proper formula for this ionic compound is mgo. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Magnesium + chlorine = magnesium chloride. Mg + cl = mgcl2 is a synthesis reaction where one. Now consider the. Chlorine And Magnesium Ionic Compound.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine And Magnesium Ionic Compound Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Therefore, the proper formula for this ionic compound is mgo. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. The periodic table can help us recognize many of the compounds that are ionic: Two chlorine. Chlorine And Magnesium Ionic Compound.
From www.echemi.com
What is the formula of the ionic compound formed from magnesium, mg Chlorine And Magnesium Ionic Compound Mg + cl = mgcl2 is a synthesis reaction where one. Now consider the ionic compound formed by magnesium and chlorine. The periodic table can help us recognize many of the compounds that are ionic: Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. Two. Chlorine And Magnesium Ionic Compound.
From www.fishersci.nl
Magnesium chloride, pure, ACROS Organics™ Other Compounds Chlorine And Magnesium Ionic Compound When a metal is combined with one or more nonmetals, the. Mg + cl = mgcl2 is a synthesis reaction where one. The periodic table can help us recognize many of the compounds that are ionic: Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Magnesium + chlorine = magnesium. Chlorine And Magnesium Ionic Compound.
From montessorimuddle.org
An Introduction to Ionic Bonding Montessori Muddle Chlorine And Magnesium Ionic Compound The periodic table can help us recognize many of the compounds that are ionic: Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Therefore, the proper formula for this ionic compound is mgo. Now consider. Chlorine And Magnesium Ionic Compound.
From schematicsetwall.z14.web.core.windows.net
Correct Electron Dot Diagram For Magnesium Chlorine And Magnesium Ionic Compound Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. Now consider the ionic compound formed by magnesium and chlorine. The attraction between the oppositely charged ions forms the ionic bond between. Mg + cl = mgcl2 is a synthesis reaction where one. Answer a based. Chlorine And Magnesium Ionic Compound.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Chlorine And Magnesium Ionic Compound Two chlorine atoms will each gain one electron from the magnesium atom. Now consider the ionic compound formed by magnesium and chlorine. Magnesium + chlorine = magnesium chloride. The periodic table can help us recognize many of the compounds that are ionic: Therefore, the proper formula for this ionic compound is mgo. Answer a based on the combinations listed in. Chlorine And Magnesium Ionic Compound.
From mavink.com
Magnesium And Hcl Reaction Chlorine And Magnesium Ionic Compound Now consider the ionic compound formed by magnesium and chlorine. When a metal is combined with one or more nonmetals, the. Magnesium + chlorine = magnesium chloride. Therefore, the proper formula for this ionic compound is mgo. Two chlorine atoms will each gain one electron from the magnesium atom. Mg + cl = mgcl2 is a synthesis reaction where one.. Chlorine And Magnesium Ionic Compound.
From www.youtube.com
Is MgCl2 (Magnesium chloride) Ionic or Covalent? YouTube Chlorine And Magnesium Ionic Compound Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. Now consider the ionic compound formed by magnesium and chlorine. The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the. Magnesium. Chlorine And Magnesium Ionic Compound.
From courses.lumenlearning.com
Lewis Symbols and Structures Chemistry for Majors Chlorine And Magnesium Ionic Compound Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. Mg +. Chlorine And Magnesium Ionic Compound.
From www.elektramagnesium.com.au
Ionic Magnesium Chloride Hexahydrate or Chelated Magnesium? Elektra Chlorine And Magnesium Ionic Compound Magnesium + chlorine = magnesium chloride. Now consider the ionic compound formed by magnesium and chlorine. The attraction between the oppositely charged ions forms the ionic bond between. Therefore, the proper formula for this ionic compound is mgo. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Two chlorine atoms will each gain one. Chlorine And Magnesium Ionic Compound.
From www.youtube.com
Equation for MgCl2 + H2O (Magnesium chloride + Water) YouTube Chlorine And Magnesium Ionic Compound Two chlorine atoms will each gain one electron from the magnesium atom. Now consider the ionic compound formed by magnesium and chlorine. When a metal is combined with one or more nonmetals, the. The periodic table can help us recognize many of the compounds that are ionic: A magnesium ion has a 2+ charge, while a chlorine ion has a. Chlorine And Magnesium Ionic Compound.
From tutorsuhu.com
What Is The Molecular Structure Of Magnesium Chloride Tutor Suhu Chlorine And Magnesium Ionic Compound Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. The periodic table can help us recognize many of the compounds that are ionic: Answer a based on the combinations listed. Chlorine And Magnesium Ionic Compound.
From www.elevise.co.uk
C2 A) Ionic Bonds AQA Combined Science Trilogy Elevise Chlorine And Magnesium Ionic Compound Magnesium + chlorine = magnesium chloride. Therefore, the proper formula for this ionic compound is mgo. The attraction between the oppositely charged ions forms the ionic bond between. Mg + cl = mgcl2 is a synthesis reaction where one. When a metal is combined with one or more nonmetals, the. Answer a based on the combinations listed in section 3.2,. Chlorine And Magnesium Ionic Compound.
From www.freeexamacademy.com
Atoms, Elements And Compounds Free Exam Academy Chlorine And Magnesium Ionic Compound Magnesium + chlorine = magnesium chloride. Mg + cl = mgcl2 is a synthesis reaction where one. The attraction between the oppositely charged ions forms the ionic bond between. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Therefore, the proper formula for this ionic compound is mgo. Now consider the ionic compound formed. Chlorine And Magnesium Ionic Compound.
From slidesharenow.blogspot.com
How Does An Ionic Bond Form Between Sodium And Chlorine slideshare Chlorine And Magnesium Ionic Compound Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Now consider the ionic compound formed by magnesium and chlorine. Two chlorine atoms will each gain one. Chlorine And Magnesium Ionic Compound.
From www.slideshare.net
Chem matters ch6_ionic_bond Chlorine And Magnesium Ionic Compound Two chlorine atoms will each gain one electron from the magnesium atom. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Therefore, the proper formula for this ionic compound is mgo. Magnesium + chlorine = magnesium chloride. Now consider the ionic compound formed by magnesium and chlorine. A magnesium ion. Chlorine And Magnesium Ionic Compound.
From www.numerade.com
SOLVED Use the electron dot structures to determine chemical formulas Chlorine And Magnesium Ionic Compound Now consider the ionic compound formed by magnesium and chlorine. Therefore, the proper formula for this ionic compound is mgo. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Magnesium + chlorine = magnesium chloride. The periodic table can help us recognize many of the compounds that are ionic: Answer a based on the. Chlorine And Magnesium Ionic Compound.
From www.gauthmath.com
Solved Magnesium and chlorine react to form an ionic compound. What is Chlorine And Magnesium Ionic Compound When a metal is combined with one or more nonmetals, the. Two chlorine atoms will each gain one electron from the magnesium atom. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Therefore, the proper formula for this ionic compound is mgo. The periodic table can help us recognize many. Chlorine And Magnesium Ionic Compound.
From www.jagranjosh.com
What are Ionic Compounds and how they are formed? Chlorine And Magnesium Ionic Compound Now consider the ionic compound formed by magnesium and chlorine. The periodic table can help us recognize many of the compounds that are ionic: Mg + cl = mgcl2 is a synthesis reaction where one. The attraction between the oppositely charged ions forms the ionic bond between. Magnesium + chlorine = magnesium chloride. When a metal is combined with one. Chlorine And Magnesium Ionic Compound.
From www.edplace.com
Explain Ionic bonding Worksheet EdPlace Chlorine And Magnesium Ionic Compound The periodic table can help us recognize many of the compounds that are ionic: Therefore, the proper formula for this ionic compound is mgo. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Magnesium + chlorine = magnesium chloride. Mg + cl = mgcl2 is a synthesis reaction where one.. Chlorine And Magnesium Ionic Compound.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine And Magnesium Ionic Compound Therefore, the proper formula for this ionic compound is mgo. Now consider the ionic compound formed by magnesium and chlorine. The attraction between the oppositely charged ions forms the ionic bond between. Two chlorine atoms will each gain one electron from the magnesium atom. The periodic table can help us recognize many of the compounds that are ionic: Magnesium +. Chlorine And Magnesium Ionic Compound.
From www.youtube.com
Draw the Lewis Structure of MgCl2 (magnesium chloride) YouTube Chlorine And Magnesium Ionic Compound The attraction between the oppositely charged ions forms the ionic bond between. The periodic table can help us recognize many of the compounds that are ionic: A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,.. Chlorine And Magnesium Ionic Compound.
From quizizz.com
Properties of Ionic Compounds Chemistry Quiz Quizizz Chlorine And Magnesium Ionic Compound Two chlorine atoms will each gain one electron from the magnesium atom. When a metal is combined with one or more nonmetals, the. Therefore, the proper formula for this ionic compound is mgo. The periodic table can help us recognize many of the compounds that are ionic: Answer a based on the combinations listed in section 3.2, these elements will. Chlorine And Magnesium Ionic Compound.
From studylib.net
Ionic Compounds Naming Chlorine And Magnesium Ionic Compound When a metal is combined with one or more nonmetals, the. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Therefore, the proper formula for this ionic compound is mgo. The attraction between the oppositely charged ions forms the ionic bond between. Two chlorine atoms will each gain one electron. Chlorine And Magnesium Ionic Compound.
From www.numerade.com
SOLVED Determine whether the following pairs of elements can form Chlorine And Magnesium Ionic Compound The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the. Magnesium + chlorine = magnesium chloride. Therefore, the proper formula for this ionic compound is mgo. Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of. Chlorine And Magnesium Ionic Compound.
From www.thesciencehive.co.uk
Mass and Mole Calculations (AQA) — the science hive Chlorine And Magnesium Ionic Compound Therefore, the proper formula for this ionic compound is mgo. Mg + cl = mgcl2 is a synthesis reaction where one. Now consider the ionic compound formed by magnesium and chlorine. Two chlorine atoms will each gain one electron from the magnesium atom. Answer a based on the combinations listed in section 3.2, these elements will combine to form an. Chlorine And Magnesium Ionic Compound.
From www.shutterstock.com
16 Magnesium Chloride Element Stock Vectors and Vector Art Shutterstock Chlorine And Magnesium Ionic Compound Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. When a metal is combined with one or more nonmetals, the. Magnesium + chlorine = magnesium chloride. Now consider the ionic compound formed by magnesium and chlorine. A magnesium ion has a 2+ charge, while a chlorine ion has a 1−. Chlorine And Magnesium Ionic Compound.
From www.chemicalslearning.com
Magnesium Chloride Formula, Properties and Uses Chlorine And Magnesium Ionic Compound Now consider the ionic compound formed by magnesium and chlorine. Mg + cl = mgcl2 is a synthesis reaction where one. The periodic table can help us recognize many of the compounds that are ionic: Therefore, the proper formula for this ionic compound is mgo. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:.. Chlorine And Magnesium Ionic Compound.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Chlorine And Magnesium Ionic Compound Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. Two chlorine atoms will each gain one electron from the magnesium atom. Magnesium + chlorine = magnesium chloride. Mg + cl = mgcl2 is a synthesis reaction where one. A magnesium ion has a 2+ charge,. Chlorine And Magnesium Ionic Compound.
From revisechemistry.uk
Chemical Bonds, Ionic, Covalent and Metallic AQA C2 revisechemistry.uk Chlorine And Magnesium Ionic Compound The attraction between the oppositely charged ions forms the ionic bond between. Mg + cl = mgcl2 is a synthesis reaction where one. Now consider the ionic compound formed by magnesium and chlorine. The periodic table can help us recognize many of the compounds that are ionic: When a metal is combined with one or more nonmetals, the. Answer a. Chlorine And Magnesium Ionic Compound.
From chem.libretexts.org
Ionic Solids Chemistry LibreTexts Chlorine And Magnesium Ionic Compound Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Magnesium + chlorine = magnesium chloride. When a metal is combined with one or more nonmetals, the. Two chlorine atoms will each gain one electron from the magnesium atom. Mg + cl = mgcl2 is a synthesis reaction where one. A. Chlorine And Magnesium Ionic Compound.
From blog.iceslicer.com
Chloride Spotlight What is Magnesium Chloride? Chlorine And Magnesium Ionic Compound When a metal is combined with one or more nonmetals, the. Answer a based on the combinations listed in section 3.2, these elements will combine to form an ionic compound,. Mg + cl = mgcl2 is a synthesis reaction where one. Two chlorine atoms will each gain one electron from the magnesium atom. Magnesium + chlorine = magnesium chloride. Now. Chlorine And Magnesium Ionic Compound.
From www.youtube.com
How to draw dot and cross diagram of magnesium chloride ionic compound Chlorine And Magnesium Ionic Compound Two chlorine atoms will each gain one electron from the magnesium atom. The attraction between the oppositely charged ions forms the ionic bond between. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Now consider the ionic compound formed by magnesium and chlorine. Because the electronegativity of chlorine is higher than the electronegativity of. Chlorine And Magnesium Ionic Compound.
From www.slideshare.net
Ionic bonding Chlorine And Magnesium Ionic Compound Because the electronegativity of chlorine is higher than the electronegativity of sodium, chlorine will attract the valence electron of the sodium atom very strongly. A magnesium ion has a 2+ charge, while a chlorine ion has a 1− charge:. Therefore, the proper formula for this ionic compound is mgo. The attraction between the oppositely charged ions forms the ionic bond. Chlorine And Magnesium Ionic Compound.