Zinc Chloride And Potassium Sulfide Empirical Formula . There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. Click the card to flip 👆. Steps to determine empirical formula: Figure 10.61 zns, zinc sulfide (or zinc blende). The formula unit or empirical formula represents the. Remember that for the metal elements in groups 1, 2, and 3 the. Each individual pair called a formula unit or empirical formula. A macroscopic sample is composed of myriads of nacl pairs; What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Assume a \ (100 \: The formula of an ionic compound can be deduced from the formulae of its ions. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. \text {g}\) sample of the compound so that the given percentages can be directly.
from www.numerade.com
Remember that for the metal elements in groups 1, 2, and 3 the. Figure 10.61 zns, zinc sulfide (or zinc blende). \text {g}\) sample of the compound so that the given percentages can be directly. The formula unit or empirical formula represents the. The formula of an ionic compound can be deduced from the formulae of its ions. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. A macroscopic sample is composed of myriads of nacl pairs; Assume a \ (100 \: Click the card to flip 👆.
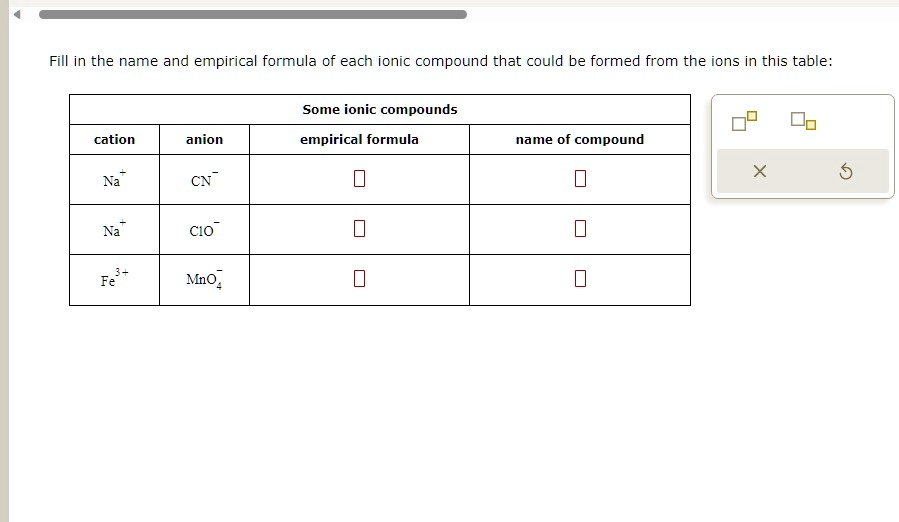
SOLVED Texts Fill in the name and empirical formula of each ionic
Zinc Chloride And Potassium Sulfide Empirical Formula Each individual pair called a formula unit or empirical formula. A macroscopic sample is composed of myriads of nacl pairs; Click the card to flip 👆. \text {g}\) sample of the compound so that the given percentages can be directly. The formula of an ionic compound can be deduced from the formulae of its ions. Remember that for the metal elements in groups 1, 2, and 3 the. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Each individual pair called a formula unit or empirical formula. Figure 10.61 zns, zinc sulfide (or zinc blende). The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. The formula unit or empirical formula represents the. Assume a \ (100 \: For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. Steps to determine empirical formula:
From www.numerade.com
SOLVED solution A solution B Does a precipitate form when A and B are Zinc Chloride And Potassium Sulfide Empirical Formula The formula of an ionic compound can be deduced from the formulae of its ions. Remember that for the metal elements in groups 1, 2, and 3 the. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. The formula unit or empirical formula represents the. A macroscopic. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Zinc Chloride And Potassium Sulfide Empirical Formula The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. The formula of an ionic compound can be deduced from the formulae of its ions. Each individual pair called a formula unit or empirical formula. \text {g}\). Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED 1. Can you determine the molecular formula of a substance from Zinc Chloride And Potassium Sulfide Empirical Formula The formula of an ionic compound can be deduced from the formulae of its ions. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Click the card. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.chegg.com
Complete The Table Below By Deciding Whether Aqueo... Zinc Chloride And Potassium Sulfide Empirical Formula There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. Click the card to flip 👆. The formula of an ionic compound can be deduced from the formulae of its ions. Remember that for the metal elements in groups 1, 2, and 3 the. For compounds containing only monatomic ions (such as. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.chegg.com
Solved O CHEMICAL REACTIONS Predicting precipitation Zinc Chloride And Potassium Sulfide Empirical Formula Click the card to flip 👆. Each individual pair called a formula unit or empirical formula. The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. Remember that for the metal elements in groups 1, 2, and. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.slideserve.com
PPT Writing Ionic Formulas PowerPoint Presentation, free download Zinc Chloride And Potassium Sulfide Empirical Formula A macroscopic sample is composed of myriads of nacl pairs; For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Each individual pair called a formula unit or empirical formula. Figure 10.61 zns, zinc sulfide (or zinc blende). The formula unit or empirical formula represents the. The objective of this. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.coursehero.com
[Solved] The chemical formula for potassium sulfide is KSO 3 OK2503 Zinc Chloride And Potassium Sulfide Empirical Formula For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. The formula unit or empirical formula represents the. Click the card to flip 👆. A macroscopic sample is composed of myriads of nacl pairs; What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix. Zinc Chloride And Potassium Sulfide Empirical Formula.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Empirical Formula A macroscopic sample is composed of myriads of nacl pairs; Steps to determine empirical formula: For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Click the card to flip 👆. Remember that for the metal elements in groups 1, 2, and 3 the. What is the empirical formula of. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Empirical Formula Problems empirical formula for the following Zinc Chloride And Potassium Sulfide Empirical Formula Remember that for the metal elements in groups 1, 2, and 3 the. Click the card to flip 👆. Figure 10.61 zns, zinc sulfide (or zinc blende). A macroscopic sample is composed of myriads of nacl pairs; The formula of an ionic compound can be deduced from the formulae of its ions. Assume a \ (100 \: Each individual pair. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.youtube.com
How to Write the Formula for Zinc sulfide (ZnS) YouTube Zinc Chloride And Potassium Sulfide Empirical Formula The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. The formula of an ionic compound can be deduced from the formulae of its ions. Click the card to flip 👆. A macroscopic sample is composed of. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Part A Zinc Sulfide A student performed an experiment to Zinc Chloride And Potassium Sulfide Empirical Formula The formula unit or empirical formula represents the. Figure 10.61 zns, zinc sulfide (or zinc blende). Each individual pair called a formula unit or empirical formula. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. The formula of an ionic compound can be deduced from the formulae. Zinc Chloride And Potassium Sulfide Empirical Formula.
From brainly.in
Just write the chemical formula of these compounds/radicals1 Zinc Chloride And Potassium Sulfide Empirical Formula What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Steps to determine empirical formula: Each individual pair called a formula unit or empirical formula. Click the card to flip 👆. \text {g}\) sample of the compound so that the given percentages can be directly. A macroscopic sample. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Names of ionic compounds Formula K2S BaF2 MgO Na3N AlCl3 Mg3P2 Zinc Chloride And Potassium Sulfide Empirical Formula Each individual pair called a formula unit or empirical formula. The formula of an ionic compound can be deduced from the formulae of its ions. The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. Figure 10.61. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.chegg.com
Solved REPORT SHEET Chemical Formulas A, Zinc Chloride 1. Zinc Chloride And Potassium Sulfide Empirical Formula Remember that for the metal elements in groups 1, 2, and 3 the. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Assume a \ (100 \: Click the card to flip 👆. The formula of an ionic compound can be deduced from the formulae of its. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Texts Fill in the name and empirical formula of each ionic Zinc Chloride And Potassium Sulfide Empirical Formula Click the card to flip 👆. Each individual pair called a formula unit or empirical formula. Steps to determine empirical formula: The formula unit or empirical formula represents the. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. What is the empirical formula of the precipitate formed when zinc. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED 'What is the empirical formula of zinc chloride? Show Zinc Chloride And Potassium Sulfide Empirical Formula Steps to determine empirical formula: Assume a \ (100 \: The formula unit or empirical formula represents the. A macroscopic sample is composed of myriads of nacl pairs; \text {g}\) sample of the compound so that the given percentages can be directly. Each individual pair called a formula unit or empirical formula. Remember that for the metal elements in groups. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Compound Name sadium chloride Formula Comoouno Name Formula Zinc Chloride And Potassium Sulfide Empirical Formula \text {g}\) sample of the compound so that the given percentages can be directly. Figure 10.61 zns, zinc sulfide (or zinc blende). There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVEDprecipitate will form enter its empirical formula in the last Zinc Chloride And Potassium Sulfide Empirical Formula There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. Remember that for the metal elements in groups 1, 2, and 3 the. Each individual pair called a formula unit or empirical formula. The objective of this and the next two sections is to teach you to write the formula for a. Zinc Chloride And Potassium Sulfide Empirical Formula.
From questions.kunduz.com
Complete the table below by deciding whe... Chemistry Zinc Chloride And Potassium Sulfide Empirical Formula The formula unit or empirical formula represents the. Steps to determine empirical formula: Remember that for the metal elements in groups 1, 2, and 3 the. Figure 10.61 zns, zinc sulfide (or zinc blende). \text {g}\) sample of the compound so that the given percentages can be directly. There are four zinc ions and four sulfide ions in the unit. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Texts Fill in the name and empirical formula of each ionic Zinc Chloride And Potassium Sulfide Empirical Formula What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Steps to determine empirical formula: \text {g}\) sample of the compound so that the given percentages can be directly. There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. Click. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED CHEMICAL REACTIONS Predicting precipitation nplete the table Zinc Chloride And Potassium Sulfide Empirical Formula There are four zinc ions and four sulfide ions in the unit cell, giving the empirical formula zns. Click the card to flip 👆. Steps to determine empirical formula: Assume a \ (100 \: What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. A macroscopic sample is. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.chegg.com
Solved solution A solution B Does a precipitate form when A Zinc Chloride And Potassium Sulfide Empirical Formula The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. Steps to determine empirical formula: Figure 10.61 zns, zinc sulfide (or zinc blende). A macroscopic sample is composed of myriads of nacl pairs; For compounds containing only. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Complete the table below by deciding whether a precipitate Zinc Chloride And Potassium Sulfide Empirical Formula For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. \text {g}\) sample of the compound so that the given percentages can be directly. Remember that for the metal elements in groups 1, 2, and 3 the. The objective of this and the next two sections is to teach you. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Formulas of Ionic Compounds Positive ion Negative ion Formula Zinc Chloride And Potassium Sulfide Empirical Formula The formula of an ionic compound can be deduced from the formulae of its ions. \text {g}\) sample of the compound so that the given percentages can be directly. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. The objective of this and the next two sections. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.slideserve.com
PPT Nomenclature writing chemical formulas naming chemical compounds Zinc Chloride And Potassium Sulfide Empirical Formula A macroscopic sample is composed of myriads of nacl pairs; The formula of an ionic compound can be deduced from the formulae of its ions. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Figure 10.61 zns, zinc sulfide (or zinc blende). Click the card to flip 👆. \text. Zinc Chloride And Potassium Sulfide Empirical Formula.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Empirical Formula Steps to determine empirical formula: The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. A macroscopic sample is composed of myriads of nacl pairs; \text {g}\) sample of the compound so that the given percentages can. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.slideserve.com
PPT 10 Write formulas for compounds formed from these pairs of ions Zinc Chloride And Potassium Sulfide Empirical Formula The formula of an ionic compound can be deduced from the formulae of its ions. Assume a \ (100 \: A macroscopic sample is composed of myriads of nacl pairs; For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Click the card to flip 👆. \text {g}\) sample of. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Does Silver Nitrate and Potassium Sulfide precipitate when Zinc Chloride And Potassium Sulfide Empirical Formula For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. Click the card to flip 👆. Each individual pair called a formula unit or empirical formula. Steps to determine empirical formula: A macroscopic sample is composed of myriads of nacl pairs; The formula unit or empirical formula represents the. The. Zinc Chloride And Potassium Sulfide Empirical Formula.
From lauriekyrran.blogspot.com
Potassium Hydroxide And Iron Ii Nitrate Precipitate LaurieKyrran Zinc Chloride And Potassium Sulfide Empirical Formula For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such as caso 4),. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Remember that for the metal elements in groups 1, 2, and 3 the. Steps to determine empirical formula:. Zinc Chloride And Potassium Sulfide Empirical Formula.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Empirical Formula Each individual pair called a formula unit or empirical formula. The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. For compounds containing only monatomic ions (such as nacl) and for many compounds containing polyatomic ions (such. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Does a empirical formula of precipitate precipitate form when A Zinc Chloride And Potassium Sulfide Empirical Formula Remember that for the metal elements in groups 1, 2, and 3 the. The formula unit or empirical formula represents the. A macroscopic sample is composed of myriads of nacl pairs; \text {g}\) sample of the compound so that the given percentages can be directly. Assume a \ (100 \: For compounds containing only monatomic ions (such as nacl) and. Zinc Chloride And Potassium Sulfide Empirical Formula.
From exoquepbq.blob.core.windows.net
Potassium Sulfide Zinc Chloride at Gerry Muniz blog Zinc Chloride And Potassium Sulfide Empirical Formula \text {g}\) sample of the compound so that the given percentages can be directly. A macroscopic sample is composed of myriads of nacl pairs; The objective of this and the next two sections is to teach you to write the formula for a simple inorganic compound from its name—and vice versa—and introduce you to some of the. What is the. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Complete the table below bv deciding whether precipitate forms Zinc Chloride And Potassium Sulfide Empirical Formula The formula unit or empirical formula represents the. Each individual pair called a formula unit or empirical formula. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Click the card to flip 👆. Assume a \ (100 \: The objective of this and the next two sections. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED Complete the table below by deciding whether precipitate forms Zinc Chloride And Potassium Sulfide Empirical Formula Click the card to flip 👆. The formula unit or empirical formula represents the. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. Remember that for the metal elements in groups 1, 2, and 3 the. Assume a \ (100 \: The formula of an ionic compound. Zinc Chloride And Potassium Sulfide Empirical Formula.
From www.numerade.com
SOLVED 'Does a empirical formula of precipitate precipitate form when Zinc Chloride And Potassium Sulfide Empirical Formula \text {g}\) sample of the compound so that the given percentages can be directly. What is the empirical formula of the precipitate formed when zinc chloride and potassium sulfide mix together your solution’s ready to go!. A macroscopic sample is composed of myriads of nacl pairs; There are four zinc ions and four sulfide ions in the unit cell, giving. Zinc Chloride And Potassium Sulfide Empirical Formula.