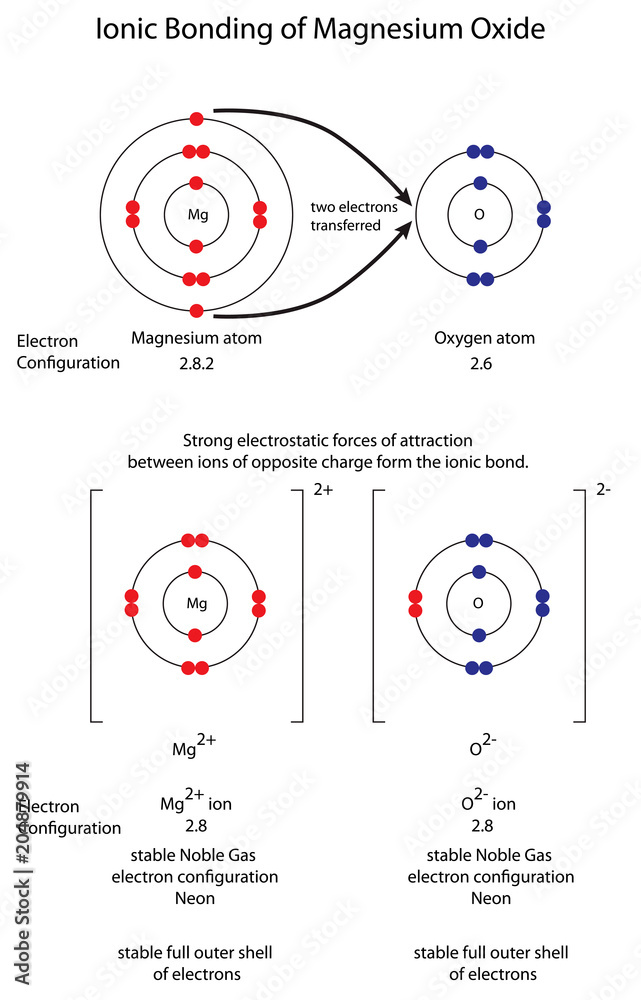
Electron Configuration Of Magnesium In Excited State . 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The ground state electron configuration of magnesium is important in understanding its chemical properties. Since 1s can only hold two electrons. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level.
from ar.inspiredpencil.com
Since 1s can only hold two electrons. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. The ground state electron configuration of magnesium is important in understanding its chemical properties. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level.
Electron Configuration Of Magnesium
Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. Since 1s can only hold two electrons. The ground state electron configuration of magnesium is important in understanding its chemical properties. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the.
From www.animalia-life.club
Magnesium Electron Configuration Electron Configuration Of Magnesium In Excited State Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. The ground state electron configuration of magnesium is important in understanding its. Electron Configuration Of Magnesium In Excited State.
From general.chemistrysteps.com
Orbital Diagrams Chemistry Steps Electron Configuration Of Magnesium In Excited State An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons. 19 rows to determine the electron configuration of a particular atom, start at the nucleus. Electron Configuration Of Magnesium In Excited State.
From www.transtutors.com
(Solved) The Highest Energy Electron Of Magnesium Is Excited To The Electron Configuration Of Magnesium In Excited State Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. An electron configuration representing an atom in the excited state. Electron Configuration Of Magnesium In Excited State.
From www.nagwa.com
Question Video Identifying the Energy Level Diagram That Represents Electron Configuration Of Magnesium In Excited State An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in. Electron Configuration Of Magnesium In Excited State.
From www.thesciencehive.co.uk
The Periodic Table (GCSE) — the science hive Electron Configuration Of Magnesium In Excited State Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. The ground state electron configuration of magnesium is important in understanding its chemical properties. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. What is the electron. Electron Configuration Of Magnesium In Excited State.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium In Excited State The ground state electron configuration of magnesium is important in understanding its chemical properties. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. Since 1s can only hold two electrons. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus,. Electron Configuration Of Magnesium In Excited State.
From www.numerade.com
SOLVED Base your answers to questions 3032 on the information below Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. In writing the electron configuration. Electron Configuration Of Magnesium In Excited State.
From www.youtube.com
Magnesium electronic configuration How to Write Magnesium electronic Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr. Electron Configuration Of Magnesium In Excited State.
From www.slideserve.com
PPT Electron Configuration Ions and Excite State PowerPoint Electron Configuration Of Magnesium In Excited State In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in. Electron Configuration Of Magnesium In Excited State.
From utedzz.blogspot.com
Periodic Table Magnesium Atomic Number Periodic Table Timeline Electron Configuration Of Magnesium In Excited State What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. 19 rows to determine the electron configuration of a particular atom, start. Electron Configuration Of Magnesium In Excited State.
From mungfali.com
Magnesium Orbital Diagram Electron Configuration Of Magnesium In Excited State An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus,. Electron Configuration Of Magnesium In Excited State.
From commons.wikimedia.org
FileElectron shell 012 magnesium.png Wikimedia Commons Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. What is the electron configuration for an. Electron Configuration Of Magnesium In Excited State.
From www.slideserve.com
PPT Introductory Chemistry , 2 nd Edition Nivaldo Tro PowerPoint Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. The ground state electron configuration of magnesium is important in understanding its chemical properties. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in. Electron Configuration Of Magnesium In Excited State.
From www.youtube.com
Mg Orbital Diagram How to Write the Atomic Orbital Diagram for Electron Configuration Of Magnesium In Excited State An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. The ground state electron configuration of magnesium is important in understanding its chemical properties. Since 1s can only hold two electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium. Electron Configuration Of Magnesium In Excited State.
From www.numerade.com
SOLVEDWhat is a groundstate electron configuration? What is an Electron Configuration Of Magnesium In Excited State An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. The ground state electron configuration of magnesium is important in understanding its chemical properties. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons. 19. Electron Configuration Of Magnesium In Excited State.
From general.chemistrysteps.com
Electron Configurations of Ions Chemistry Steps Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. The ground state electron configuration. Electron Configuration Of Magnesium In Excited State.
From sciencenotes.org
List of Electron Configurations of Elements Electron Configuration Of Magnesium In Excited State 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)#. Electron Configuration Of Magnesium In Excited State.
From www.newtondesk.com
Magnesium Mg (Elements 12) of Periodic Table Elements FlashCards Electron Configuration Of Magnesium In Excited State What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is. Electron Configuration Of Magnesium In Excited State.
From www.youtube.com
Electronic configuration for Magnesium (Mg) spdf Trick Chemistry Electron Configuration Of Magnesium In Excited State What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and. Electron Configuration Of Magnesium In Excited State.
From www.youtube.com
Electron Configuration of Magnesium Mg Lesson YouTube Electron Configuration Of Magnesium In Excited State In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. Since 1s can only hold two electrons. The ground state electron configuration of magnesium is important in understanding its chemical properties. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in. Electron Configuration Of Magnesium In Excited State.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium In Excited State The ground state electron configuration of magnesium is important in understanding its chemical properties. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. Since 1s can only hold two electrons. What. Electron Configuration Of Magnesium In Excited State.
From slidetodoc.com
Aim What happens when electrons get excited Do Electron Configuration Of Magnesium In Excited State What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. The ground state electron configuration of magnesium is important in understanding its chemical properties. In writing. Electron Configuration Of Magnesium In Excited State.
From www.toppr.com
Write the electron dot structure of magnesium and oxygen. Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. An electron configuration representing an atom in the excited state will show a. Electron Configuration Of Magnesium In Excited State.
From www.youtube.com
Mg 2+ Electron Configuration (Magnesium Ion) YouTube Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. The ground state electron configuration of magnesium. Electron Configuration Of Magnesium In Excited State.
From utedzz.blogspot.com
Periodic Table Magnesium Electron Configuration Periodic Table Timeline Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. The ground state electron configuration of magnesium is important in understanding its chemical properties. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. An electron configuration representing an atom in the excited state will show a valence. Electron Configuration Of Magnesium In Excited State.
From www.numerade.com
SOLVED a) Write down the electronic configuration of magnesium. b Electron Configuration Of Magnesium In Excited State In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is. Electron Configuration Of Magnesium In Excited State.
From ar.inspiredpencil.com
Electron Configuration Of Magnesium Electron Configuration Of Magnesium In Excited State An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and. Electron Configuration Of Magnesium In Excited State.
From www.slideserve.com
PPT Ground vs. Excited State PowerPoint Presentation ID313264 Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only. Electron Configuration Of Magnesium In Excited State.
From periodictable.me
Magnesium Electron Configuration (Mg) with Orbital Diagram Electron Configuration Of Magnesium In Excited State In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron,. Electron Configuration Of Magnesium In Excited State.
From www.vectorstock.com
Diagram representation of the element magnesium Vector Image Electron Configuration Of Magnesium In Excited State 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. Since 1s can only hold two electrons. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. An electron configuration. Electron Configuration Of Magnesium In Excited State.
From ar.inspiredpencil.com
Electron Configuration Magnesium Electron Configuration Of Magnesium In Excited State 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. The ground state electron configuration of magnesium is important in understanding its chemical properties. Since 1s can only. Electron Configuration Of Magnesium In Excited State.
From www.pinterest.com
Excited State Easy Science Easy science, Electron configuration, Ap Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. Magnesium starts off with a ground state configuration of #[ne]3s^2# #ul(uarr darr)# #3s# thus, only a #3s# electron, which is highest in energy in the. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. An electron configuration representing an atom in the excited state will. Electron Configuration Of Magnesium In Excited State.
From www.webelements.com
Elements Periodic Table » Magnesium » properties of free atoms Electron Configuration Of Magnesium In Excited State Since 1s can only hold two electrons. In writing the electron configuration for magnesium the first two electrons will go in the 1s orbital. An electron configuration representing an atom in the excited state will show a valence electron promoted to a higher energy level. 19 rows to determine the electron configuration of a particular atom, start at the nucleus. Electron Configuration Of Magnesium In Excited State.
From www.youtube.com
Magnesium Ground State Electron Configuration YouTube Electron Configuration Of Magnesium In Excited State What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. The ground state electron configuration of magnesium is important in understanding its. Electron Configuration Of Magnesium In Excited State.
From www.pinterest.se
GensonScience Magnesium Atom model project, Atom model, Atoms and Electron Configuration Of Magnesium In Excited State 19 rows to determine the electron configuration of a particular atom, start at the nucleus and add electrons one by one until the number of. What is the electron configuration for an excited atom of phosphorous, ready to emit a photon of energy and return to ground state?. An electron configuration representing an atom in the excited state will show. Electron Configuration Of Magnesium In Excited State.