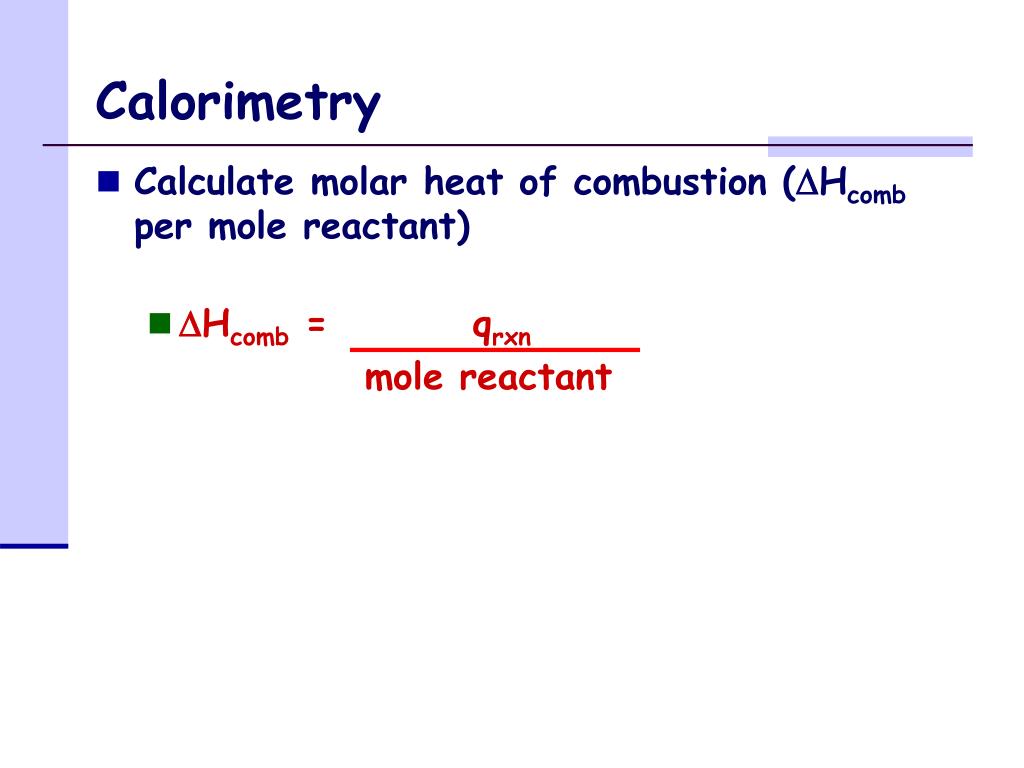
Calorimetry Calculations Chemistry . Apply the first law of thermodynamics to calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. Calculate and interpret heat and related properties using typical calorimetry data; When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Explain the technique of calorimetry; Calculate and interpret heat and related properties using typical calorimetry data To use calorimetric data to calculate. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. How to calculate a calorimeter constant. Additionally, it can find the. Explain the technique of calorimetry; It can analyze the heat exchange between up to 3 objects. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction.
from www.slideserve.com
Explain the technique of calorimetry; Calculate and interpret heat and related properties using typical calorimetry data To use calorimetric data to calculate. How to calculate a calorimeter constant. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. The calorimetry calculator can help you solve complex calorimetry problems. Explain the technique of calorimetry; Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Apply the first law of thermodynamics to calorimetry.
PPT Calorimetry PowerPoint Presentation, free download ID6133898
Calorimetry Calculations Chemistry In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. It can analyze the heat exchange between up to 3 objects. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first law of thermodynamics to calorimetry. How to calculate a calorimeter constant. Explain the technique of calorimetry; The calorimetry calculator can help you solve complex calorimetry problems. Calculate and interpret heat and related properties using typical calorimetry data Calculate and interpret heat and related properties using typical calorimetry data; Additionally, it can find the. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. To use calorimetric data to calculate. Explain the technique of calorimetry;
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation, free download ID6133898 Calorimetry Calculations Chemistry Apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data; Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction.. Calorimetry Calculations Chemistry.
From www.showme.com
4 steps for solving calorimetry problems Science, Chemistry ShowMe Calorimetry Calculations Chemistry In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Additionally, it can find the. Calculate and interpret heat and related properties using typical calorimetry data Calculate and interpret heat and related properties using typical calorimetry data; Compare heat flow from hot to cold. Calorimetry Calculations Chemistry.
From studylib.net
Calorimetry Worksheet Calorimetry Calculations Chemistry It can analyze the heat exchange between up to 3 objects. Explain the technique of calorimetry; The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. Calculate and interpret heat and related properties using typical calorimetry data; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. In this article,. Calorimetry Calculations Chemistry.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data; To use calorimetric data to calculate. Explain the technique of calorimetry; Calculate and interpret heat and related properties using typical calorimetry data Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of. Calorimetry Calculations Chemistry.
From quizmischances.z4.web.core.windows.net
How To Calculate Calorimetry Calorimetry Calculations Chemistry Explain the technique of calorimetry; Explain the technique of calorimetry; How to calculate a calorimeter constant. The calorimetry calculator can help you solve complex calorimetry problems. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. It can analyze the heat exchange between up. Calorimetry Calculations Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data; When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. How to calculate a calorimeter constant. It can analyze the heat exchange between up to 3 objects. In this article, you will learn about calorimetry and calorimeters, mainly used. Calorimetry Calculations Chemistry.
From www.youtube.com
CHEMISTRY 101 Constant Pressure Calorimetry YouTube Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Apply the first law of thermodynamics to calorimetry. Additionally, it can find the. Explain. Calorimetry Calculations Chemistry.
From www.studocu.com
Calorimetry Lab Calorimetry Calculations Heat Capacity of Calorimetry Calculations Chemistry It can analyze the heat exchange between up to 3 objects. Apply the first law of thermodynamics to calorimetry. Explain the technique of calorimetry; Additionally, it can find the. Explain the technique of calorimetry; Calculate and interpret heat and related properties using typical calorimetry data; In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific. Calorimetry Calculations Chemistry.
From www.youtube.com
AP Chemistry Thermochemical Equations and Calorimetry YouTube Calorimetry Calculations Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Explain the technique of calorimetry; It can analyze the heat exchange between up to 3 objects. Calculate and interpret heat and related properties using typical calorimetry data; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Apply the first. Calorimetry Calculations Chemistry.
From www.youtube.com
Using Calorimetry to Calculate Enthalpies of Reaction Chemistry Calorimetry Calculations Chemistry Explain the technique of calorimetry; Explain the technique of calorimetry; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. To use calorimetric data. Calorimetry Calculations Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Change in Internal Energy Using Constant Calorimetry Calculations Chemistry In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Additionally, it can find the. Calculate and interpret heat and related properties using typical calorimetry data; Calculate and interpret heat and related properties using typical calorimetry data It can analyze the heat exchange between. Calorimetry Calculations Chemistry.
From www.youtube.com
How to find calorimeter constant YouTube Calorimetry Calculations Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Additionally, it can find the. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Apply the first law of thermodynamics to calorimetry. How to calculate a calorimeter constant. Explain the technique of calorimetry; In this article, you will learn. Calorimetry Calculations Chemistry.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Calculations Chemistry In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Additionally, it can find the. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. How to calculate a calorimeter constant. When 40.0 ml of water at 60.0 °c. Calorimetry Calculations Chemistry.
From www.gbu-presnenskij.ru
Chapter 25 Essential Calorimetry Formulae James Kennedy, 47 OFF Calorimetry Calculations Chemistry Explain the technique of calorimetry; How to calculate a calorimeter constant. The calorimetry calculator can help you solve complex calorimetry problems. To use calorimetric data to calculate. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calculate and interpret heat and related properties using typical calorimetry data; Calculate and interpret heat and related properties. Calorimetry Calculations Chemistry.
From www.wizeprep.com
Calorimetry Wize University Chemistry Textbook Wizeprep Calorimetry Calculations Chemistry It can analyze the heat exchange between up to 3 objects. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. How to calculate a calorimeter constant. Apply the first law of thermodynamics to calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. Additionally, it can find the. Explain the technique of calorimetry;. Calorimetry Calculations Chemistry.
From quizdbbarefooted.z21.web.core.windows.net
How To Calculate Calorimeter Calorimetry Calculations Chemistry In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Explain the technique of calorimetry; It can analyze the heat exchange between up to 3 objects. To use calorimetric data to calculate. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimetry Calculations Chemistry.
From users.highland.edu
Calorimetry Calorimetry Calculations Chemistry Explain the technique of calorimetry; To use calorimetric data to calculate. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Calculate and interpret heat and related properties using typical calorimetry data Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the. Calorimetry Calculations Chemistry.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data; How to calculate a calorimeter constant. Calculate and interpret heat and related properties using typical calorimetry data Additionally, it can find the. It can analyze the heat exchange between up to 3 objects. Explain the technique of calorimetry; When 40.0 ml of water at 60.0 °c is added to. Calorimetry Calculations Chemistry.
From studylib.net
Calorimetry Calorimetry Calculations Chemistry Additionally, it can find the. Apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data The calorimetry calculator can help you solve complex calorimetry problems. Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Explain the technique of calorimetry; In this article, you will learn. Calorimetry Calculations Chemistry.
From www.youtube.com
AP Chemistry Thermochemistry I Part 4 Constant Pressure Calorimetry Calorimetry Calculations Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. The calorimetry calculator can help you solve complex calorimetry problems. Apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data Explain the technique of calorimetry; Additionally, it can find the. How to calculate a calorimeter constant.. Calorimetry Calculations Chemistry.
From acechemistry.co.uk
Calorimetry ALevel chemistry to calculate enthalpy change Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Explain the technique of calorimetry; In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Explain the technique. Calorimetry Calculations Chemistry.
From www.chegg.com
Calculate the calorimeter constant (From Lab) So for Calorimetry Calculations Chemistry It can analyze the heat exchange between up to 3 objects. Calculate and interpret heat and related properties using typical calorimetry data Explain the technique of calorimetry; When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Compare heat flow from hot to cold objects in an ideal calorimeter versus. Calorimetry Calculations Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID1609320 Calorimetry Calculations Chemistry It can analyze the heat exchange between up to 3 objects. Explain the technique of calorimetry; When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Calculate and interpret heat and related properties using typical calorimetry data; Apply the first law of thermodynamics to calorimetry. To use calorimetric data to. Calorimetry Calculations Chemistry.
From www.slideserve.com
PPT Unit 13 Thermochemistry PowerPoint Presentation, free download Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data Additionally, it can find the. Explain the technique of calorimetry; Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. The calorimetry calculator can help you solve complex calorimetry problems. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at. Calorimetry Calculations Chemistry.
From www.slideserve.com
PPT Calorimetry PowerPoint Presentation ID6133898 Calorimetry Calculations Chemistry Explain the technique of calorimetry; Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Apply the first law of thermodynamics to calorimetry. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. The calorimetry calculator can help you. Calorimetry Calculations Chemistry.
From www.youtube.com
Calorimetry (AQA A level Chemistry) YouTube Calorimetry Calculations Chemistry When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or. Calorimetry Calculations Chemistry.
From www.youtube.com
Calorimeter Calculations m value explained! OCR A Level Chemistry Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data; In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Explain the technique of calorimetry; To use calorimetric data to calculate. Apply the first law of thermodynamics to calorimetry. Calorimetry measures enthalpy changes. Calorimetry Calculations Chemistry.
From www.youtube.com
Final Temperature Calorimetry Practice Problems Chemistry YouTube Calorimetry Calculations Chemistry Additionally, it can find the. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Explain the technique of calorimetry; The calorimetry calculator can help you solve complex calorimetry problems. Explain the technique of calorimetry; Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. How to calculate a calorimeter. Calorimetry Calculations Chemistry.
From studylib.net
Calorimetry Worksheet Calorimetry Calculations Chemistry Additionally, it can find the. Explain the technique of calorimetry; Apply the first law of thermodynamics to calorimetry. The calorimetry calculator can help you solve complex calorimetry problems. To use calorimetric data to calculate. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction.. Calorimetry Calculations Chemistry.
From saylordotorg.github.io
Calorimetry Calorimetry Calculations Chemistry Calculate and interpret heat and related properties using typical calorimetry data It can analyze the heat exchange between up to 3 objects. To use calorimetric data to calculate. Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Additionally, it can find the. Explain the technique of calorimetry; Compare heat flow from hot to cold. Calorimetry Calculations Chemistry.
From www.studocu.com
Calorimetry Calculations Notes 2020 copy In this lesson we will learn Calorimetry Calculations Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Additionally, it can find the. How to calculate a calorimeter constant. Apply the first law of thermodynamics to calorimetry. When 40.0 ml of water at 60.0 °c is added to 40.0 ml at 25.0 °c water already in a. Calculate and interpret heat and related. Calorimetry Calculations Chemistry.
From www.youtube.com
How To Solve Basic Calorimetry Problems in Chemistry YouTube Calorimetry Calculations Chemistry It can analyze the heat exchange between up to 3 objects. Additionally, it can find the. To use calorimetric data to calculate. Explain the technique of calorimetry; The calorimetry calculator can help you solve complex calorimetry problems. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical. Calorimetry Calculations Chemistry.
From www.youtube.com
CHEMISTRY 101 Calculating Heat Capacity of a Bomb Calorimeter YouTube Calorimetry Calculations Chemistry How to calculate a calorimeter constant. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Apply the first law of thermodynamics to calorimetry. Calculate and interpret heat and related properties using typical calorimetry data When 40.0 ml of water at 60.0 °c is. Calorimetry Calculations Chemistry.
From www.youtube.com
Calorimetry Part 2 calculations YouTube Calorimetry Calculations Chemistry Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. Calculate and interpret heat and related properties using typical calorimetry data; In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal changes in a physical or chemical reaction. Additionally, it can find the. How to calculate. Calorimetry Calculations Chemistry.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Calculations Chemistry Compare heat flow from hot to cold objects in an ideal calorimeter versus a real. Calculate and interpret heat and related properties using typical calorimetry data Calorimetry measures enthalpy changes during chemical processes, where the magnitude of the temperature change depends. In this article, you will learn about calorimetry and calorimeters, mainly used to calculate specific heat capacity and thermal. Calorimetry Calculations Chemistry.