Presentation Of Medicinal Products . The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. If the main physical mode of.
from www.slideserve.com
Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. If the main physical mode of. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products.
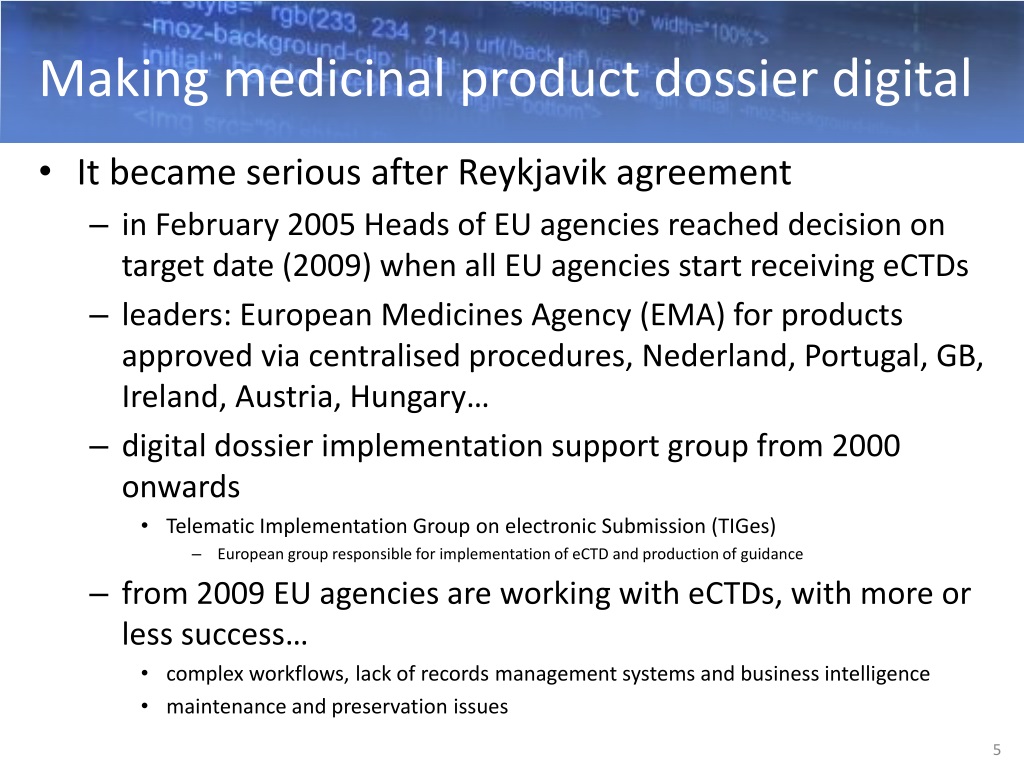
PPT Ph.D. Arian Rajh Agency for Medicinal Products and Medical
Presentation Of Medicinal Products Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. If the main physical mode of. The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Guideline on the readability of the labelling and package leaflet of medicinal products for human use.
From blog.logodesignguru.com
A to Z Branding Style Guide For Your Healthcare Startup Think Design Presentation Of Medicinal Products The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. If the main physical. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Herbal Medicinal Products Market PowerPoint Presentation, free Presentation Of Medicinal Products This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. The pharmaceutical. Presentation Of Medicinal Products.
From www.slideserve.com
PPT HERBAL MEDICINE PowerPoint Presentation, free download ID2705807 Presentation Of Medicinal Products Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. If the main physical mode of. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Identification of Medicinal Products & Pharmacovigilance Task Presentation Of Medicinal Products Guideline on the readability of the labelling and package leaflet of medicinal products for human use. If the main physical mode of. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. The pharmaceutical form of a medicinal product should be described by a single full standard term of the european. Presentation Of Medicinal Products.
From www.grc-health.com
Investigational Medicinal Product labelling an overview — GRCHealth Presentation Of Medicinal Products This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Volume 10 of the publication the rules governing medicinal products in. Presentation Of Medicinal Products.
From thummimng.com
Presentation, Preparation and Standardisation of Herbal Medicinal Presentation Of Medicinal Products This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. Guideline on the readability of the labelling and package. Presentation Of Medicinal Products.
From www.vecteezy.com
Pharmaceutical Production Infographic Set 483585 Vector Art at Vecteezy Presentation Of Medicinal Products Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. A device that achieves. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Product Labels PowerPoint Presentation, free download ID2034676 Presentation Of Medicinal Products Guideline on the readability of the labelling and package leaflet of medicinal products for human use. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. This page lists documents related to data submission for authorised medicines, including the. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Ph.D. Arian Rajh Agency for Medicinal Products and Medical Presentation Of Medicinal Products The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. A device that achieves its intended. Presentation Of Medicinal Products.
From www.slideserve.com
PPT 4. Clinical Trials Supply Investigational Medicinal Products Presentation Of Medicinal Products Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. If the main physical mode of. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of. Presentation Of Medicinal Products.
From www.slideteam.net
Pharmaceutical Product Lifecycle Pharmaceutical Development New Presentation Of Medicinal Products A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. The pharmaceutical form of a medicinal. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Product Labels PowerPoint Presentation, free download ID2034676 Presentation Of Medicinal Products A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Volume 10 of the. Presentation Of Medicinal Products.
From www.health2wellnessblog.com
Top 10 Benefits of Using Herbal Medicines Health2wellness Presentation Of Medicinal Products The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. Volume 2 of the publications the rules governing. Presentation Of Medicinal Products.
From www.sketchbubble.com
Pharmaceutical Product Development PowerPoint and Google Slides Template Presentation Of Medicinal Products Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. This. Presentation Of Medicinal Products.
From www.slideegg.com
Editable herbal medicine PPT Template and Google Slides Presentation Of Medicinal Products This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. Guideline on the scientific application and the practical arrangements. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Medicinal products dossier PowerPoint Presentation, free download Presentation Of Medicinal Products If the main physical mode of. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed. Presentation Of Medicinal Products.
From epthinktank.eu
Medicinal products in the European Union The legal framework for Presentation Of Medicinal Products The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance. Presentation Of Medicinal Products.
From www.slidegeeks.com
Picture Of Medicinal Drugs Ppt PowerPoint Presentation Styles Themes Presentation Of Medicinal Products Guideline on the readability of the labelling and package leaflet of medicinal products for human use. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically. Presentation Of Medicinal Products.
From powerpoint-templates.digitalofficepro.com
Herbal medicine powerpoint template PowerPoint Template Herbal Presentation Of Medicinal Products The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Guideline on the scientific application and the. Presentation Of Medicinal Products.
From www.slideegg.com
Herbal Medicine PowerPoint Templates & Google Slides Presentation Of Medicinal Products The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Guideline on the scientific. Presentation Of Medicinal Products.
From www.slideegg.com
Get Herbal Medicine PowerPoint Templates Free Slide Design Presentation Of Medicinal Products Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. A device. Presentation Of Medicinal Products.
From pharmaceutical-journal.com
Advanced therapy medicinal products a comprehensive overview for Presentation Of Medicinal Products Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Guideline on the scientific application and. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Clinical Trials and Pharmacy PowerPoint Presentation, free Presentation Of Medicinal Products Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. The pharmaceutical form of. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Product Labels PowerPoint Presentation, free download ID2034676 Presentation Of Medicinal Products Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. If the main physical mode of.. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Structure of Dossier of Medicinal Product Q part PowerPoint Presentation Of Medicinal Products The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. If the main physical mode of. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product.. Presentation Of Medicinal Products.
From www.slideegg.com
Health Supplements PPT Presentation Template & Google Slides Presentation Of Medicinal Products Guideline on the readability of the labelling and package leaflet of medicinal products for human use. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. If the main physical mode of. Volume. Presentation Of Medicinal Products.
From docslib.org
A Guide to What Is a Medicinal Product DocsLib Presentation Of Medicinal Products Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Guideline on. Presentation Of Medicinal Products.
From onedollargraphics.com
Pharmaceutical Medicine Packaging Design & MockUp PSD Presentation Of Medicinal Products Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. If the main physical mode of. This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. Guideline on the readability of the labelling and package leaflet of medicinal products for human. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Advanced Therapy Medicinal Products ATMPs PowerPoint Presentation Of Medicinal Products This page lists documents related to data submission for authorised medicines, including the legal notice, detailed guidance documents. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. Volume 10 of the publication the rules governing medicinal products in the european union contains guidance documents applying to. Guideline on the readability of the. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Herbal Medicinal Products Market PowerPoint Presentation, free Presentation Of Medicinal Products The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Volume 10 of the publication the rules. Presentation Of Medicinal Products.
From pharmaceutical-journal.com
Advanced therapy medicinal products a comprehensive overview for Presentation Of Medicinal Products A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Guideline on the readability of the. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Homeopathic medicinal product PowerPoint Presentation, free Presentation Of Medicinal Products If the main physical mode of. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal product. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Volume 2 of the publications the rules. Presentation Of Medicinal Products.
From creativemarket.com
Herbal Medicine Label Presentation Templates Creative Market Presentation Of Medicinal Products Guideline on the readability of the labelling and package leaflet of medicinal products for human use. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. The ema coordinates the assessment of the quality, safety and efficacy of medicinal products. If the main physical mode of. This page lists documents related. Presentation Of Medicinal Products.
From www.vecteezy.com
Label pack for medicinal tablets, label medicine paper design, medicine Presentation Of Medicinal Products The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. Guideline on the scientific application and the practical arrangements necessary to implement regulation (ec) no 507/2006 on the conditional. Guideline on the readability of the labelling and package leaflet of medicinal products for human use. A device. Presentation Of Medicinal Products.
From www.slideserve.com
PPT Structure of Dossier of Medicinal Product Q part PowerPoint Presentation Of Medicinal Products Volume 2 of the publications the rules governing medicinal products in the european union contains a list of regulatory guidelines related. The pharmaceutical form of a medicinal product should be described by a single full standard term of the european pharmacopoeia using the plural. A device that achieves its intended medical purpose pharmacologically, immunologically, or metabolically qualifies as a medicinal. Presentation Of Medicinal Products.