Therapeutic Products Act . Health products (therapeutic products) regulations 2016. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. In exercise of the powers conferred by sections 71 and 72 of the health. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence If you need more information about this act, please contact the administering agency:
from www.swissmedic.ch
The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. Health products (therapeutic products) regulations 2016. In exercise of the powers conferred by sections 71 and 72 of the health. If you need more information about this act, please contact the administering agency:
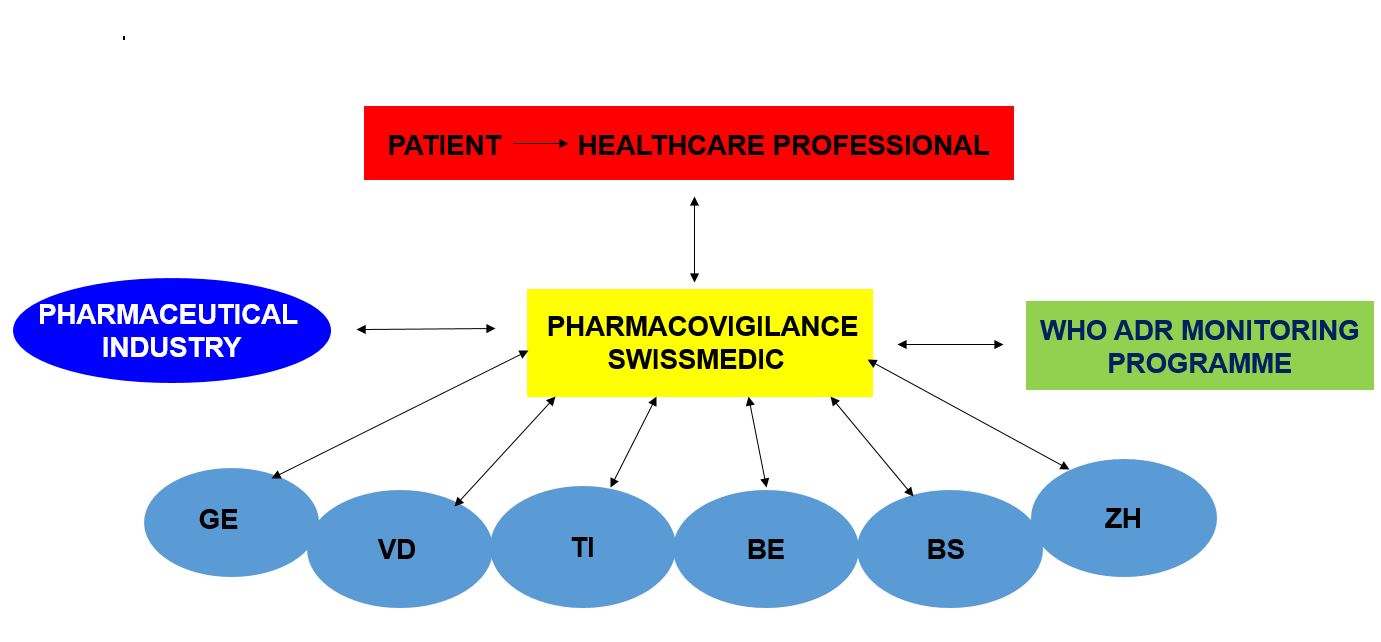
Pharmacovigilance
Therapeutic Products Act An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence In exercise of the powers conferred by sections 71 and 72 of the health. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. If you need more information about this act, please contact the administering agency: You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. Health products (therapeutic products) regulations 2016.
From healthynaturalsystems.com
NuTherapy Products Healthy Natural Solutions Therapeutic Products Act Health products (therapeutic products) regulations 2016. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. In. Therapeutic Products Act.
From www.newshub.co.nz
Patient safety advocate Carmel Berry 'horrified' at Government's plan Therapeutic Products Act If you need more information about this act, please contact the administering agency: The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence Health products (therapeutic products) regulations 2016. An act to regulate the manufacture, import, supply, presentation. Therapeutic Products Act.
From nztech.org.nz
Therapeutic Products Act seminar NZTech Therapeutic Products Act In exercise of the powers conferred by sections 71 and 72 of the health. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. The therapeutic products act 2023 (new. Therapeutic Products Act.
From www.conqueringchd.org
Patient Engagement Tool The Care Partnership Pyramid Conquering CHD Therapeutic Products Act The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the.. Therapeutic Products Act.
From www.swissmedic.ch
Pharmacovigilance Therapeutic Products Act In exercise of the powers conferred by sections 71 and 72 of the health. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. If you need more information about this act, please contact the administering agency: Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence The. Therapeutic Products Act.
From www.naturalhealthalliance.co.nz
Media Release Therapeutic Products Act Natural Health Alliance Therapeutic Products Act Health products (therapeutic products) regulations 2016. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and. Therapeutic Products Act.
From multi-clean.com
MultiClean How to eliminate odors using BioEnzymatic MultiClean Therapeutic Products Act In exercise of the powers conferred by sections 71 and 72 of the health. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. If you need more information about this act, please contact the administering agency: Health products (therapeutic products) regulations 2016. An act to regulate the manufacture, import, supply,. Therapeutic Products Act.
From www.garymoller.com
Good news! The Therapeutic Products Act is one step closer to being Therapeutic Products Act Health products (therapeutic products) regulations 2016. If you need more information about this act, please contact the administering agency: In exercise of the powers conferred by sections 71 and 72 of the health. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Part 7 exceptions — manufacture, import and wholesale. Therapeutic Products Act.
From www.itsuptous.org
Visual Breakdown of the CARES Act and its Economic Impact UpToUs Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Health products (therapeutic products) regulations 2016. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import,. Therapeutic Products Act.
From www.researchgate.net
(PDF) Consideration of and expectations for the Pharmaceuticals Therapeutic Products Act In exercise of the powers conferred by sections 71 and 72 of the health. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence The health products act (hpa) provides for the legislative basis for regulating the manufacture,. Therapeutic Products Act.
From www.garymoller.com
The Therapeutic Products Act (TPA) A Threat to Free Speech on Therapeutic Products Act An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. In exercise of the powers conferred by sections 71 and 72 of the health. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Health products (therapeutic products) regulations 2016.. Therapeutic Products Act.
From dailytelegraph.co.nz
Therapeutic Products Act repeal bill gets 1st reading Daily Telegraph NZ Therapeutic Products Act The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Health products (therapeutic products) regulations 2016. If you need more information about this act, please contact the administering agency: In exercise. Therapeutic Products Act.
From www.homeopathbarbara.nz
Petition for the Therapeutic Products Act Homeopath Barbara Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. If you need more information about this act, please contact the administering agency: Health products (therapeutic products) regulations 2016. In exercise of the powers conferred by sections 71 and 72 of the health. An act to regulate the manufacture, import, supply,. Therapeutic Products Act.
From bookstore.gpo.gov
Approved Drug Products With Therapeutic Equivalence Evaluations, 37th Therapeutic Products Act The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the.. Therapeutic Products Act.
From www.naturalhealthalliance.co.nz
Media Release NHA the repeal of Therapeutic Products Act Therapeutic Products Act An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. If you need more information about this act, please contact the administering agency: You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. In exercise of the powers conferred by. Therapeutic Products Act.
From www.elocal.co.nz
Therapeutic Products Act Government legislates in favour of Big Therapeutic Products Act The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. In. Therapeutic Products Act.
From www.cmdt.org.nz
What the Therapeutic Products Act will mean for medical device Therapeutic Products Act The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence An act to regulate the manufacture, import, supply,. Therapeutic Products Act.
From www.minterellison.co.nz
It’s official new Therapeutic Products Act to replace Medicines Act Therapeutic Products Act Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. In exercise of the powers conferred by sections 71 and 72 of the health. If you need more information about this act, please contact the administering agency: An. Therapeutic Products Act.
From mindfuel.co.nz
Therapeutic Products Bill Submissions Due Sunday March 5th MindFuel Therapeutic Products Act If you need more information about this act, please contact the administering agency: Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. The therapeutic products act 2023 (new act) has now passed into law, marking the most. Therapeutic Products Act.
From www.regulatoryaffairsnews.com
USFDA's Draft Guidance Orange Book Questions and Answers Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. Health products (therapeutic products) regulations 2016. The therapeutic products act 2023 (new act) has now passed into law, marking the. Therapeutic Products Act.
From studylib.net
Part 7 Advertising Of Therapeutic Products Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. In exercise of the powers conferred by sections 71 and 72 of the health. The therapeutic products act 2023 (new. Therapeutic Products Act.
From www.linkedin.com
Duncan Cotterill on LinkedIn The Therapeutic Products Act 2023 has Therapeutic Products Act Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence Health products (therapeutic products) regulations 2016. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. In exercise of the powers conferred by sections 71 and 72 of the health. You will need to register your therapeutic products. Therapeutic Products Act.
From www.cooneyleesmorgan.co.nz
Regulatory update Repeal of the Therapeutic Product Act Articles Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. The. Therapeutic Products Act.
From www.thelawyermag.com
Associate health minister announces repeal of Therapeutic Products Act Therapeutic Products Act The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. If you need more information about this act, please contact the administering agency: The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. An act to regulate the manufacture, import, supply,. Therapeutic Products Act.
From www.homeopathbarbara.nz
Therapeutic Products Act Homeopath Barbara Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. An act. Therapeutic Products Act.
From www.naturalhealthalliance.co.nz
Join the fight Repeal the Therapeutic Products Act Natural Health Therapeutic Products Act If you need more information about this act, please contact the administering agency: You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. In exercise of the powers conferred by sections. Therapeutic Products Act.
From www.johner-institute.nz
New Zealand Therapeutic Products Act 2023 and Medical Devices Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. In exercise of the powers conferred by sections 71 and 72 of the health. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. Part 7 exceptions — manufacture, import and. Therapeutic Products Act.
From www.minterellison.co.nz
It’s official new Therapeutic Products Act to replace Medicines Act Therapeutic Products Act The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. If you need more information about this act, please contact the administering agency: The health products act (hpa) provides. Therapeutic Products Act.
From www.bignewsnetwork.com
Therapeutic Products Act repeal bill gets 1st reading Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. In exercise of the powers conferred by sections 71 and 72 of the health. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence The therapeutic products act 2023 (new act) has now passed into law, marking the. Therapeutic Products Act.
From www.reddit.com
The Final Nail in the Coffin? Therapeutic Products Act passed under Therapeutic Products Act In exercise of the powers conferred by sections 71 and 72 of the health. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. An act to regulate the manufacture, import,. Therapeutic Products Act.
From www.garymoller.com
The Therapeutic Products Act (TPA) A Threat to Free Speech on Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Health products (therapeutic products) regulations 2016. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. The health products act (hpa) provides for the legislative basis for regulating the manufacture,. Therapeutic Products Act.
From www.garymoller.com
The Therapeutic Products Act (TPA) A Threat to Free Speech on Therapeutic Products Act The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. Part 7 exceptions — manufacture, import and wholesale of therapeutic products without licence In exercise of the powers conferred by sections 71. Therapeutic Products Act.
From www.canada.ca
Classification of products under the Food and Drugs Act (F&DA) Canada.ca Therapeutic Products Act The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. In exercise of the powers conferred by sections 71 and 72 of the health. Health products (therapeutic products) regulations 2016. If you need more information about this act, please contact the administering agency: You will need to register your therapeutic. Therapeutic Products Act.
From dailytelegraph.co.nz
Therapeutic Products Act to be repealed Daily Telegraph NZ Therapeutic Products Act The health products act (hpa) provides for the legislative basis for regulating the manufacture, import, supply, presentation and advertisement. You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. The therapeutic products act 2023 (new act) has now passed into law, marking the most significant change to new zealand’s. In exercise. Therapeutic Products Act.
From exportertoday.co.nz
Therapeutic Products Act repeal means export exemption more urgent than Therapeutic Products Act You will need to register your therapeutic products and apply for a licence before you import, manufacture or wholesale. In exercise of the powers conferred by sections 71 and 72 of the health. An act to regulate the manufacture, import, supply, presentation and advertisement of health products and of active ingredients used in the. Health products (therapeutic products) regulations 2016.. Therapeutic Products Act.