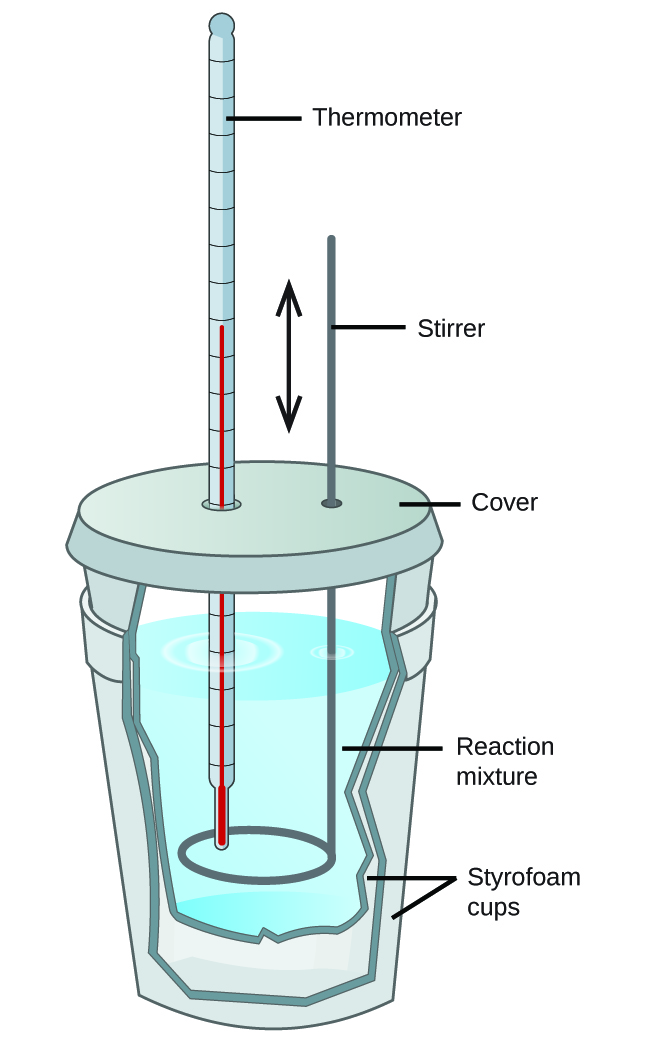
Calorimetry Set Up . One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Most students will already know that a spoon in a foam cup of cocoa gets hot but the cup does not because heat is transferred more easily to the spoon. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. How to do a simple calorimeter experiment. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). Calorimetry is used to measure amounts of heat transferred to. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from wisc.pb.unizin.org
One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. How to do a simple calorimeter experiment. The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Most students will already know that a spoon in a foam cup of cocoa gets hot but the cup does not because heat is transferred more easily to the spoon. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information.
5.2 Calorimetry Chemistry
Calorimetry Set Up The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). Calorimetry is used to measure amounts of heat transferred to. Most students will already know that a spoon in a foam cup of cocoa gets hot but the cup does not because heat is transferred more easily to the spoon. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. How to do a simple calorimeter experiment. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is.
From www.savemyexams.com
Calorimetry Experiments SL IB Chemistry Revision Notes 2025 Save My Calorimetry Set Up A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. If heat is absorbed or enters the system, the. Calorimetry Set Up.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry Set Up.
From www.hanlin.com
IB DP Chemistry SL复习笔记5.1.4 Calorimetry Experiments翰林国际教育 Calorimetry Set Up A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is also made of an insulated cup that limits the heat lost from the system. Calorimetry Set Up.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. One technique we can use to measure the amount of heat involved in a. Calorimetry Set Up.
From wisc.pb.unizin.org
5.2 Calorimetry Chemistry Calorimetry Set Up A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is used to measure amounts of heat transferred to. The amount. Calorimetry Set Up.
From 2012books.lardbucket.org
Calorimetry Calorimetry Set Up A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. Calorimetry is used to measure amounts of. Calorimetry Set Up.
From www.expii.com
Bomb Calorimeter — Structure & Function Expii Calorimetry Set Up A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. One technique we can use to measure the amount of heat involved. Calorimetry Set Up.
From www.slideserve.com
PPT Other important components of diet PowerPoint Presentation, free Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. How to do a simple calorimeter experiment. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. A calorimeter is a device used to. Calorimetry Set Up.
From www.pinterest.com
Calorimetry Bomb Calorimeter Experiment Science fair, Homeschool and Calorimetry Set Up A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. Calorimetry is used to measure amounts of. Calorimetry Set Up.
From www.savemyexams.co.uk
Biomass (5.3.3) AQA A Level Biology Revision Notes 2017 Save My Exams Calorimetry Set Up Most students will already know that a spoon in a foam cup of cocoa gets hot but the cup does not because heat is transferred more easily to the spoon. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the set of techniques used to. Calorimetry Set Up.
From courses.lumenlearning.com
Calorimetry Chemistry for Majors Calorimetry Set Up A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. How to do a simple calorimeter experiment. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. If heat is absorbed or enters the system, the process. Calorimetry Set Up.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Set Up A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than. Calorimetry Set Up.
From www.researchgate.net
(A) Paper cup calorimeter. (B) Student setting up the paper cup Calorimetry Set Up Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is. Calorimetry Set Up.
From www.youtube.com
Basic Calorimetry SetUp YouTube Calorimetry Set Up Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup.. Calorimetry Set Up.
From www.shaalaa.com
Explain the construction of a calorimeter. Draw the necessary figure Calorimetry Set Up A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. A calorimeter is. Calorimetry Set Up.
From www.learnable.education
Year 11 Chemistry Practical Investigation Calorimetry Experiment Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Most students will already know that a spoon in a foam cup of cocoa gets hot but the cup does not because heat is transferred more easily to the spoon. One technique we can use to measure the. Calorimetry Set Up.
From saylordotorg.github.io
Calorimetry Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup.. Calorimetry Set Up.
From ar.inspiredpencil.com
Basic Calorimeter Calorimetry Set Up How to do a simple calorimeter experiment. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). A calorimeter is generally used to measure the amount of. Calorimetry Set Up.
From www.researchgate.net
(a) Scheme of the autoclave calorimetry setup. (b) Full view of the Calorimetry Set Up Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. How to do a simple calorimeter experiment. Calorimetry is used to measure amounts of heat transferred to. If heat is absorbed or enters the. Calorimetry Set Up.
From www.youtube.com
Calorimeter Set Up and Background YouTube Calorimetry Set Up If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a. Calorimetry Set Up.
From www.tec-science.com
Calorimeter to determine the specific heat capacities of liquids tec Calorimetry Set Up If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. A calorimeter is also. Calorimetry Set Up.
From kaffee.50webs.com
Lab Calorimetry Calorimetry Set Up A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Most students will already know that a spoon in a foam cup of cocoa gets hot but the cup does not because heat is transferred more easily to the spoon. One technique we can use to measure the amount of heat. Calorimetry Set Up.
From www.youtube.com
Part I Calorimeter Set Up & Procedure YouTube Calorimetry Set Up The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). Calorimetry is used to measure amounts of heat transferred to. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. A calorimeter is a device. Calorimetry Set Up.
From www.shutterstock.com
Coffee Cup Calorimetry Setup Chemistry Diagram Stock Illustration Calorimetry Set Up How to do a simple calorimeter experiment. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. Most students. Calorimetry Set Up.
From evulpo.com
evulpo Calorimetry Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is the set. Calorimetry Set Up.
From www.youtube.com
1920 Calorimetry Lab Set Up YouTube Calorimetry Set Up If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes.. Calorimetry Set Up.
From chemistry.about.com
Coffee Cup and Bomb Calorimetry Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. Calorimetry is used to measure amounts of heat transferred to. Calorimetry is the set of techniques. Calorimetry Set Up.
From www.researchgate.net
Illustration of the calorimetry testing fixture. The setup is designed Calorimetry Set Up One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. A calorimeter is generally used to measure the amount of heat energy and then uses that. Calorimetry Set Up.
From www.chemistrystudent.com
Calorimetry (ALevel) ChemistryStudent Calorimetry Set Up Calorimetry is used to measure amounts of heat transferred to. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). One technique we can use. Calorimetry Set Up.
From www.freeastroscience.com
Exploring Calorimetry A Journey into Heat Transfer Measurement Calorimetry Set Up Most students will already know that a spoon in a foam cup of cocoa gets hot but the cup does not because heat is transferred more easily to the spoon. How to do a simple calorimeter experiment. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process. Calorimetry Set Up.
From www.vedantu.com
Bomb Calorimeter Learn Important Terms and Concepts Calorimetry Set Up A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. How to do a simple calorimeter experiment. Calorimetry is the set of techniques used to measure enthalpy changes during chemical processes. If heat is absorbed or enters the system, the process. Calorimetry Set Up.
From chem.libretexts.org
12 Calorimetry and Hess's Law (Experiment) Chemistry LibreTexts Calorimetry Set Up A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. One technique we can use to measure the amount of heat involved in a chemical or physical process is known as calorimetry. One technique we can use to measure the amount of heat involved in a chemical or physical process is. Calorimetry Set Up.
From www.britannica.com
Calorimeter Definition, Uses, Diagram, & Facts Britannica Calorimetry Set Up The amount of heat that flows into or out of the surroundings is determined with a technique called calorimetry (heat measurement). A calorimeter is generally used to measure the amount of heat energy and then uses that to calculate the specific heat of a substance or other heat related information. A calorimeter is a device used to measure the amount. Calorimetry Set Up.
From mechasource.blogspot.com
An Introduction To Calorimetry types And Uses , Bomb and Boy,s Gas Calorimetry Set Up How to do a simple calorimeter experiment. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. Calorimetry is used to measure amounts of heat transferred to. A calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.. Calorimetry Set Up.
From www.slideserve.com
PPT a “ Calorimeter ” PowerPoint Presentation, free download ID7050684 Calorimetry Set Up How to do a simple calorimeter experiment. If heat is absorbed or enters the system, the process is endothermic and if heat is evolved or exits the system, the process is. A calorimeter is also made of an insulated cup that limits the heat lost from the system even more than a regular foam cup. One technique we can use. Calorimetry Set Up.