Solubility Product Lab Report . Lab # 12 determination of the solubility product: In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. Although solubility is temperature dependent, the extent to which a substance dissolves also. Rebecca gotthelf maddy chiapperino 4/10/ 14. Determine of the solubility product constant of calcium hydroxide. The purpose of this laboratory experiment was to. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Amount of a solvent is termed its solubility. To experimentally determine the k sp of an ionic compound. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. In chm 111, we classified.
from www.studocu.com
To experimentally determine the k sp of an ionic compound. Rebecca gotthelf maddy chiapperino 4/10/ 14. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Lab # 12 determination of the solubility product: Amount of a solvent is termed its solubility. Determine of the solubility product constant of calcium hydroxide. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. The purpose of this laboratory experiment was to. In chm 111, we classified.
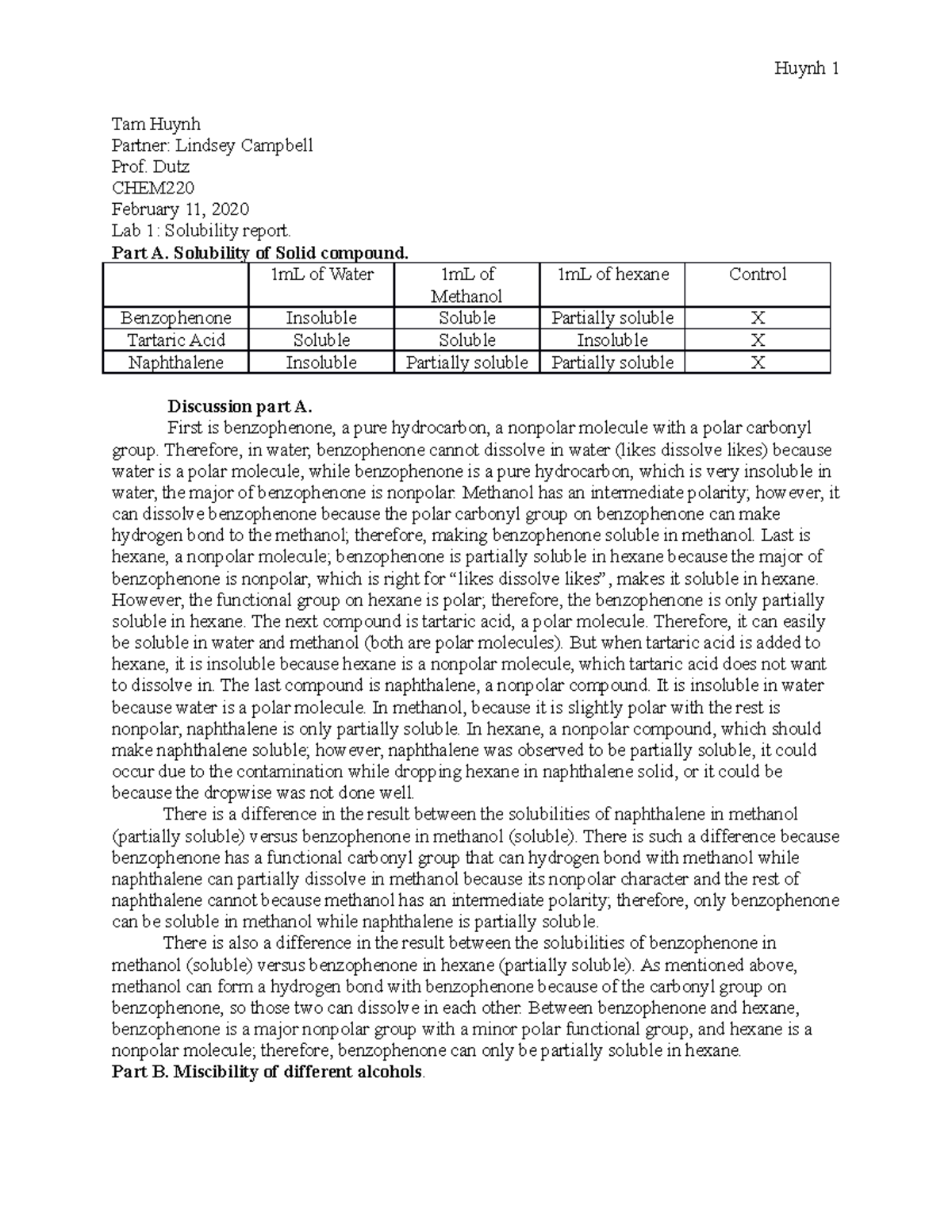
Lab Report 1 Solubility Tam Huynh Partner Lindsey Campbell Prof. Dutz CHEM February 11
Solubility Product Lab Report The purpose of this laboratory experiment was to. Amount of a solvent is termed its solubility. The purpose of this laboratory experiment was to. To experimentally determine the k sp of an ionic compound. Rebecca gotthelf maddy chiapperino 4/10/ 14. Lab # 12 determination of the solubility product: In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. In chm 111, we classified. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. Although solubility is temperature dependent, the extent to which a substance dissolves also. Determine of the solubility product constant of calcium hydroxide. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Determination of the 27 Solubility Product Lab Report Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Lab # 12 determination of the solubility product:. Solubility Product Lab Report.
From www.chegg.com
REPORT SHEET Determination of the SolubilityProduct Solubility Product Lab Report To experimentally determine the k sp of an ionic compound. Lab # 12 determination of the solubility product: The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. Amount of a solvent is termed its solubility. In chm 111, we classified. The solubility of a constituent is reliant. Solubility Product Lab Report.
From www.scribd.com
Lab Report (Solubility) PDF Solubility Solution Solubility Product Lab Report The purpose of this laboratory experiment was to. Determine of the solubility product constant of calcium hydroxide. To experimentally determine the k sp of an ionic compound. Lab # 12 determination of the solubility product: Rebecca gotthelf maddy chiapperino 4/10/ 14. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which. Solubility Product Lab Report.
From www.numerade.com
SOLVED Solubility Product Lab Report Sheet ame DATA and CALCULATIONS Concentration of the Solubility Product Lab Report Although solubility is temperature dependent, the extent to which a substance dissolves also. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. In chm 111, we classified. Amount of a solvent is termed its solubility. Determine of the solubility product constant of calcium hydroxide. To experimentally determine. Solubility Product Lab Report.
From www.studocu.com
Solubility Equilibrium Lab Report Name Zainab Hassan Partner Yi Zhang Student No 20145037 Solubility Product Lab Report Lab # 12 determination of the solubility product: In chm 111, we classified. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Rebecca gotthelf maddy chiapperino 4/10/ 14. Solubility of a salt is the quantity or amount present in some volume. Solubility Product Lab Report.
From studylib.net
Sample Lab Report Solubility Product Lab Report The purpose of this laboratory experiment was to. Rebecca gotthelf maddy chiapperino 4/10/ 14. Although solubility is temperature dependent, the extent to which a substance dissolves also. Amount of a solvent is termed its solubility. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. Determine. Solubility Product Lab Report.
From www.studocu.com
Solubility product Ksp lab report Report sheet Solubility Product Ksp Name Thi Phuoc Loan Solubility Product Lab Report Lab # 12 determination of the solubility product: Determine of the solubility product constant of calcium hydroxide. The purpose of this laboratory experiment was to. Rebecca gotthelf maddy chiapperino 4/10/ 14. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Amount of a solvent is termed its. Solubility Product Lab Report.
From www.chegg.com
REPORT SHEET EXPERIMENT Borax Solubility Solubility Product Lab Report Amount of a solvent is termed its solubility. Rebecca gotthelf maddy chiapperino 4/10/ 14. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Lab #. Solubility Product Lab Report.
From www.studocu.com
Solubility Lab Report Section W600A Date 4/14/ CHM115L Determination of a Solubility Solubility Product Lab Report In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Lab # 12 determination of the solubility product: To experimentally determine the k sp of an ionic compound. Rebecca gotthelf maddy chiapperino 4/10/ 14. In chm 111, we classified. Solubility of a salt is the quantity or amount. Solubility Product Lab Report.
From www.studocu.com
Solubility Product Of Copper Lab7Lab Report Lab 6 Determination of the Solubility Product Solubility Product Lab Report To experimentally determine the k sp of an ionic compound. Lab # 12 determination of the solubility product: The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Determine of the solubility product constant of calcium hydroxide. Although solubility is temperature dependent,. Solubility Product Lab Report.
From www.numerade.com
SOLVED LAB REPORT Prelab questions 1. Write the balanced equation for the solubility of the Solubility Product Lab Report In chm 111, we classified. The purpose of this laboratory experiment was to. Lab # 12 determination of the solubility product: In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. To experimentally determine the k sp of an ionic compound. The solubility product constant, or ksp of. Solubility Product Lab Report.
From www.scribd.com
Determination of A Solubility Product Constant Lab 19C Michelle Finkle Kenna Hunter MR Read Solubility Product Lab Report The purpose of this laboratory experiment was to. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Although solubility. Solubility Product Lab Report.
From www.chegg.com
Solved EXPERIMENT REPORT SHEET Determination of the Solubility Product Lab Report Amount of a solvent is termed its solubility. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. To experimentally determine the k sp of an ionic compound. Lab # 12 determination of the solubility product: The solubility product constant, or ksp of a compound is. Solubility Product Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Determination of the 10 Solubility Product Lab Report Rebecca gotthelf maddy chiapperino 4/10/ 14. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Solubility of a salt. Solubility Product Lab Report.
From www.studocu.com
Solubility Product Constant Lab Report Warning TT undefined function 32 Solubility Product Solubility Product Lab Report Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. The solubility product constant, or ksp of a compound is an equilibrium constant that. Solubility Product Lab Report.
From answerhappy.com
Solubility Product of Calcium lodate Lab Report Table 1. Data Summary. Molarity of Na2S₂O3 Solubility Product Lab Report In chm 111, we classified. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. Rebecca gotthelf maddy chiapperino 4/10/ 14. Although solubility is. Solubility Product Lab Report.
From www.chegg.com
Solved REPORT SHEET EXPERIMENT Determination of the Solubility Product Lab Report Although solubility is temperature dependent, the extent to which a substance dissolves also. In chm 111, we classified. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is. Solubility Product Lab Report.
From www.youtube.com
How to do lab report 007 Solubility Product Constant YouTube Solubility Product Lab Report Although solubility is temperature dependent, the extent to which a substance dissolves also. The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement. Solubility Product Lab Report.
From www.studypool.com
SOLUTION Relationship Between Temperature and Solubility Lab Report Studypool Solubility Product Lab Report Rebecca gotthelf maddy chiapperino 4/10/ 14. To experimentally determine the k sp of an ionic compound. The purpose of this laboratory experiment was to. Although solubility is temperature dependent, the extent to which a substance dissolves also. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. In. Solubility Product Lab Report.
From www.chegg.com
Laboratory Instructor EXPERIMENT 27 REPORT SHEET Solubility Product Lab Report Amount of a solvent is termed its solubility. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Although solubility is temperature dependent, the extent to which a substance dissolves also. Solubility of a salt is the quantity or amount present in. Solubility Product Lab Report.
From www.numerade.com
REPORT SHEET EXPERIMENT Determination of the SolubilityProduct Constant for a Sparingly Solubility Product Lab Report To experimentally determine the k sp of an ionic compound. Rebecca gotthelf maddy chiapperino 4/10/ 14. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. In chm 111, we classified. Determine of the solubility product constant of calcium hydroxide. The purpose of this laboratory experiment. Solubility Product Lab Report.
From www.studypool.com
SOLUTION Physical chemistry laboratory report determination of the solubility product constant Solubility Product Lab Report Amount of a solvent is termed its solubility. Determine of the solubility product constant of calcium hydroxide. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. The purpose of this laboratory experiment was to. The solubility product constant, or ksp of a compound is an. Solubility Product Lab Report.
From www.scribd.com
Solubility Lab Report Template PDF Solubility Product Lab Report Rebecca gotthelf maddy chiapperino 4/10/ 14. In chm 111, we classified. The purpose of this laboratory experiment was to. Although solubility is temperature dependent, the extent to which a substance dissolves also. The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance.. Solubility Product Lab Report.
From www.studocu.com
Lab Report Solubility Product 200.PR Department of Chemistry & Chemical Technology Studocu Solubility Product Lab Report Determine of the solubility product constant of calcium hydroxide. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. Rebecca gotthelf maddy chiapperino 4/10/ 14. The purpose of this laboratory experiment was to. In this experiment, the relative solubility (and an approximate value of the ksp). Solubility Product Lab Report.
From www.studocu.com
Experiment 8 Lab Report Solubility Product Lab Mackenzie Fusco Chem 102 Dr. Lee Hoffman Solubility Product Lab Report Rebecca gotthelf maddy chiapperino 4/10/ 14. In chm 111, we classified. To experimentally determine the k sp of an ionic compound. Lab # 12 determination of the solubility product: Determine of the solubility product constant of calcium hydroxide. Amount of a solvent is termed its solubility. The purpose of this laboratory experiment was to. The solubility of a constituent is. Solubility Product Lab Report.
From www.studypool.com
SOLUTION Physical chemistry laboratory report determination of the solubility product constant Solubility Product Lab Report Lab # 12 determination of the solubility product: Amount of a solvent is termed its solubility. Determine of the solubility product constant of calcium hydroxide. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Although solubility is temperature dependent, the extent to which a substance dissolves also.. Solubility Product Lab Report.
From studylib.net
Solubility of KNO3 Lab Report Format Solubility Product Lab Report To experimentally determine the k sp of an ionic compound. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. Amount of a solvent is termed its solubility. Although solubility is temperature dependent, the extent to which a substance dissolves also. In this experiment, the relative. Solubility Product Lab Report.
From www.studocu.com
Lab Report Effects Of Temperature On Solubility Of A Salt LAB Report 4/14/16 Effect of Studocu Solubility Product Lab Report The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Although solubility is temperature dependent, the extent to which a substance dissolves also. To experimentally determine the k sp of an ionic compound. Lab # 12 determination of the solubility product: Determine. Solubility Product Lab Report.
From www.chegg.com
Solved Experiment 9 Determination of the SolubilityProduct Solubility Product Lab Report In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Amount of a solvent is termed its solubility. The purpose of this laboratory experiment was to. Rebecca gotthelf maddy chiapperino 4/10/ 14. Determine of the solubility product constant of calcium hydroxide. The solubility of a constituent is reliant. Solubility Product Lab Report.
From www.studypool.com
SOLUTION Determination of Solubility Product Constant of Calcium Iodate Lab Studypool Solubility Product Lab Report The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Although solubility is temperature dependent, the extent to which a substance dissolves also. The purpose of this laboratory experiment was to. In chm 111, we classified. The solubility product constant, or ksp. Solubility Product Lab Report.
From www.studypool.com
SOLUTION Experiment 7 Solubility and Solubility Product Lab Report Studypool Solubility Product Lab Report The solubility product constant, or ksp of a compound is an equilibrium constant that describes the degree to which a solid dissolves. Amount of a solvent is termed its solubility. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Solubility of a salt is the quantity or. Solubility Product Lab Report.
From answerhappy.com
Solubility Product of Calcium lodate Lab Report Table 1. Data Summary. Molarity of Na2S₂O3 Solubility Product Lab Report Determine of the solubility product constant of calcium hydroxide. Although solubility is temperature dependent, the extent to which a substance dissolves also. In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Rebecca gotthelf maddy chiapperino 4/10/ 14. The solubility of a constituent is reliant on the temperature. Solubility Product Lab Report.
From www.studocu.com
Solubility Product Lab Solubility Product of Calcium Benzoate Lab Report Introduction The Solubility Product Lab Report Lab # 12 determination of the solubility product: The purpose of this laboratory experiment was to. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. To experimentally determine the k sp of an ionic compound. Determine of the solubility product constant of calcium hydroxide. Rebecca. Solubility Product Lab Report.
From www.studocu.com
Lab Report 1 Solubility Tam Huynh Partner Lindsey Campbell Prof. Dutz CHEM February 11 Solubility Product Lab Report The solubility of a constituent is reliant on the temperature of the solvent, however, how well the substance dissipates is dependent on the chemical arrangement of the substance. Rebecca gotthelf maddy chiapperino 4/10/ 14. Although solubility is temperature dependent, the extent to which a substance dissolves also. Determine of the solubility product constant of calcium hydroxide. The purpose of this. Solubility Product Lab Report.
From www.studypool.com
SOLUTION Basic Chem Lab Solubility and Solubility Product Notes + Experiment Studypool Solubility Product Lab Report In this experiment, the relative solubility (and an approximate value of the ksp) of lead iodide will be determined by direct observation. Solubility of a salt is the quantity or amount present in some volume of saturated solution, one for which an equilibrium exists between the. To experimentally determine the k sp of an ionic compound. Determine of the solubility. Solubility Product Lab Report.