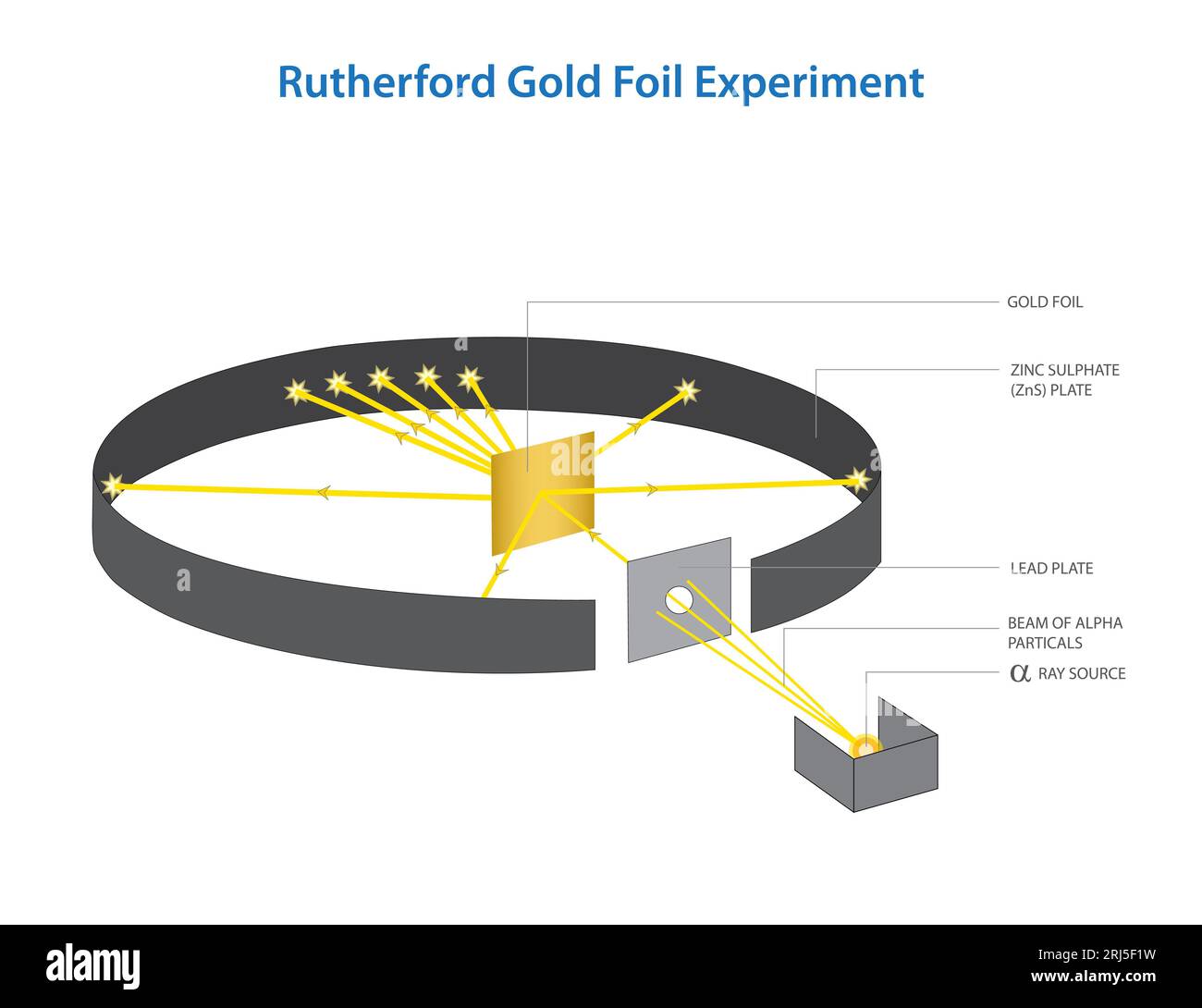
Gold Foil.experiment . gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. the gold foil experiment. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. a piece of gold foil was hit with alpha particles, which have a positive charge. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Most alpha particles went right through.
from
a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. the gold foil experiment. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Most alpha particles went right through.
Gold Foil.experiment Most alpha particles went right through. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. the gold foil experiment. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. Most alpha particles went right through. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry.
From mishiewishieblogg.blogspot.com
Chemistry Gold Foil Experiment Gold Foil.experiment the gold foil experiment. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that. Gold Foil.experiment.
From www.preparatorychemistry.com
History of Atomic Theory Gold Foil.experiment Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Most alpha particles went right through. the gold foil experiment. gold. Gold Foil.experiment.
From slideplayer.com
Anything in black letters = write it in your notes (‘knowts’) ppt Gold Foil.experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. Prior to the groundbreaking. Gold Foil.experiment.
From
Gold Foil.experiment Most alpha particles went right through. the gold foil experiment. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. a piece of gold foil was hit with alpha particles, which have a positive charge. In 1911, rutherford and coworkers hans geiger and. Gold Foil.experiment.
From
Gold Foil.experiment Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. the gold foil experiment. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. rutherford's gold foil experiment led to the discovery that most. Gold Foil.experiment.
From
Gold Foil.experiment Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. Most alpha particles went right through. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. gold was used because it was the only metal that could be rolled. Gold Foil.experiment.
From slideplayer.com
Atomic Theory. ppt download Gold Foil.experiment gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. the gold foil experiment. Most alpha particles went right through. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. In 1911,. Gold Foil.experiment.
From
Gold Foil.experiment the gold foil experiment. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. a piece of gold foil was hit with alpha particles, which have a positive. Gold Foil.experiment.
From slideplayer.com
Ch. 4 Atomic Structure 4.1 Defining the Atom. ppt download Gold Foil.experiment a piece of gold foil was hit with alpha particles, which have a positive charge. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. the gold foil experiment. Most alpha particles went right through. gold was used because it was the only metal that. Gold Foil.experiment.
From
Gold Foil.experiment a piece of gold foil was hit with alpha particles, which have a positive charge. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Prior. Gold Foil.experiment.
From
Gold Foil.experiment a piece of gold foil was hit with alpha particles, which have a positive charge. the gold foil experiment. Most alpha particles went right through. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, rutherford. Gold Foil.experiment.
From
Gold Foil.experiment Most alpha particles went right through. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be very, very thin. Gold Foil.experiment.
From
Gold Foil.experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions. Gold Foil.experiment.
From slideplayer.com
Discovery of the Atom. ppt download Gold Foil.experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Most alpha particles went right through. the gold foil experiment. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out. Gold Foil.experiment.
From
Gold Foil.experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize. Gold Foil.experiment.
From
Gold Foil.experiment gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. a piece of gold foil was hit with alpha particles, which have a positive charge. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry.. Gold Foil.experiment.
From
Gold Foil.experiment gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series. Gold Foil.experiment.
From
Gold Foil.experiment gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. the gold foil experiment. a piece of gold foil was hit with alpha particles, which have a positive charge. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions. Gold Foil.experiment.
From
Gold Foil.experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Most alpha particles went right through. gold was used because it was the only metal that. Gold Foil.experiment.
From
Gold Foil.experiment Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. rutherford's gold foil experiment led to the discovery that most of an atom's mass is. Gold Foil.experiment.
From byjus.com
Why did Rutherford select a gold foil in his αray scattering experiment? Gold Foil.experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. the gold foil experiment. Most alpha particles went right through. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. gold was used because it was the only. Gold Foil.experiment.
From
Gold Foil.experiment gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Most alpha particles went right through. Prior to the groundbreaking gold foil experiment,. Gold Foil.experiment.
From
Gold Foil.experiment the gold foil experiment. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Most alpha particles went right through. gold was used because it. Gold Foil.experiment.
From www.slideserve.com
PPT The History of the Development of the Atom PowerPoint Gold Foil.experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. Most alpha particles went right through. rutherford's gold foil experiment led to the discovery that most of an atom's. Gold Foil.experiment.
From slideplayer.com
From Antiquity to Present A Look at the History of the Atom ppt download Gold Foil.experiment Most alpha particles went right through. the gold foil experiment. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the. Gold Foil.experiment.
From
Gold Foil.experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. the gold foil experiment. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. a piece of gold foil was hit. Gold Foil.experiment.
From
Gold Foil.experiment the gold foil experiment. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. Most alpha particles went right through. gold was used because it was the only. Gold Foil.experiment.
From www.slideserve.com
PPT Chapter 4 Glow in the Dark PowerPoint Presentation, free Gold Foil.experiment Most alpha particles went right through. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. rutherford's gold foil experiment led to the discovery that most of an atom's mass is. Gold Foil.experiment.
From
Gold Foil.experiment a piece of gold foil was hit with alpha particles, which have a positive charge. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. gold was used because it was the only metal that could be rolled out to be very, very. Gold Foil.experiment.
From www.britannica.com
Ernest Rutherford Atomic Theory, & Facts Britannica Gold Foil.experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. a piece of gold foil was hit with alpha particles, which have a positive charge. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. . Gold Foil.experiment.
From slideplayer.com
Timeline Use your textbook and any other resources available to Gold Foil.experiment Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. gold was used because it was the only metal that could be. Gold Foil.experiment.
From
Gold Foil.experiment rutherford's gold foil experiment led to the discovery that most of an atom's mass is located in a dense region now called the nucleus. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. a piece of gold foil was hit with alpha particles, which have. Gold Foil.experiment.
From
Gold Foil.experiment a piece of gold foil was hit with alpha particles, which have a positive charge. the gold foil experiment. Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that. Gold Foil.experiment.
From
Gold Foil.experiment Prior to the groundbreaking gold foil experiment, rutherford was granted the nobel prize for other key contributions in the field of chemistry. the gold foil experiment. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be. Gold Foil.experiment.
From fyordzcnb.blob.core.windows.net
When Alpha Particles Are Used To Bombard Gold Foil at Josephine Gold Foil.experiment In 1911, rutherford and coworkers hans geiger and ernest marsden initiated a series of groundbreaking experiments that would. a piece of gold foil was hit with alpha particles, which have a positive charge. gold was used because it was the only metal that could be rolled out to be very, very thin without cracking. rutherford's gold foil. Gold Foil.experiment.