Air Pressure And Volume . Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Explore the experiments and equations that describe these relationships and their applications. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. See graphs, examples, and applications of this law to breathing and gas laws. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. In fact, if the volume. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.
from step1.medbullets.com
Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. In fact, if the volume. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. See graphs, examples, and applications of this law to breathing and gas laws. Explore the experiments and equations that describe these relationships and their applications. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications.
PressureVolume Curve Respiratory Medbullets Step 1
Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. See graphs, examples, and applications of this law to breathing and gas laws. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. Explore the experiments and equations that describe these relationships and their applications. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. In fact, if the volume. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure.
From derangedphysiology.com
Pressurevolume loops in the presence of lung pathology Deranged Air Pressure And Volume Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. See graphs, examples, and applications of this law to breathing and gas laws. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Boyle’s law relates the pressure and volume. Air Pressure And Volume.
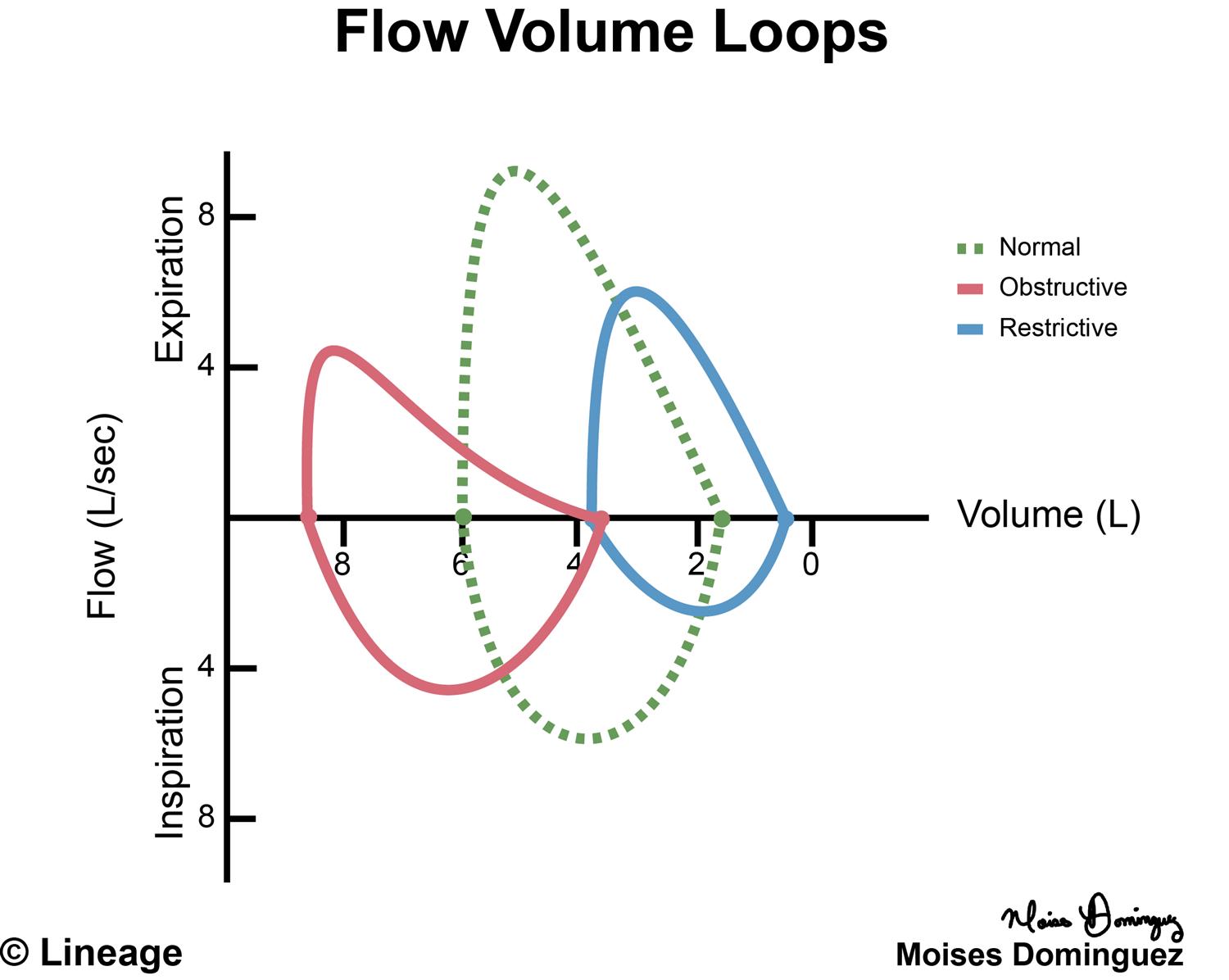
From www.slideserve.com
PPT Pressure Volume Relationship PowerPoint Presentation, free Air Pressure And Volume Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Explore the experiments and equations that describe these relationships and their applications. Boyle's law is a relation between pressure and volume of a gas. Air Pressure And Volume.
From masterconceptsinchemistry.com
What’s the relationship between pressure and volume of gas? Core Air Pressure And Volume Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. In fact, if the volume. Explore the experiments and equations that describe these relationships and their applications. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. See graphs, examples, and applications of this law to. Air Pressure And Volume.
From www.unm.edu
BIOL 238 Class Notes Respiratory System Air Pressure And Volume Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Decreasing the volume of a contained gas. Air Pressure And Volume.
From www.youtube.com
Equations of State part 1 understanding PressureVolume diagrams YouTube Air Pressure And Volume Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. In fact, if the volume. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. See graphs, examples, and applications of this law to breathing and gas laws. Boyle’s law. Air Pressure And Volume.
From pressbooks.nscc.ca
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Learn how to derive it. Air Pressure And Volume.
From www.sscadda.com
Atmospheric Pressure Air Pressure And Volume Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. See graphs, examples, and applications of this law to breathing and gas laws. In fact, if the volume. Learn how. Air Pressure And Volume.
From philschatz.com
The Process of Breathing · Anatomy and Physiology Air Pressure And Volume Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Explore the experiments and equations that describe these relationships and their applications. Learn how the volume and pressure of a gas are. Air Pressure And Volume.
From chem.libretexts.org
9.2 Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Air Pressure And Volume Explore the experiments and equations that describe these relationships and their applications. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Boyle's law is a relation between. Air Pressure And Volume.
From www.chegg.com
Solved The Pressure, Specificvolume Diagram Of An Air St... Air Pressure And Volume Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. In fact, if the volume. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and. Air Pressure And Volume.
From guidewiringdietician.z5.web.core.windows.net
Equation Relating Pressure Volume And Temp Air Pressure And Volume Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert. Air Pressure And Volume.
From www.slideserve.com
PPT The mechanics of breathing and Respiratory Volumes PowerPoint Air Pressure And Volume Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. In fact, if the volume. Boyle's law. Air Pressure And Volume.
From step1.medbullets.com
PressureVolume Curve Respiratory Medbullets Step 1 Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Explore the experiments and equations that describe these relationships and their applications. Learn how the volume and pressure of a gas are inversely proportional at constant. Air Pressure And Volume.
From www.physicsforums.com
Effect on air pressure and volume in an enclosed container Air Pressure And Volume Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. In fact, if the volume. Explore the experiments and equations that describe these relationships and their applications. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Learn how the volume and pressure of. Air Pressure And Volume.
From www.jonathandownham.com
Mechanical Ventilation Series Pressure/Volume loop….. Critical Care Air Pressure And Volume Explore the experiments and equations that describe these relationships and their applications. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. See graphs, examples, and applications of this law. Air Pressure And Volume.
From studyrocket.co.uk
Turning Forces and Pressure GCSE Physics AQA Revision Study Rocket Air Pressure And Volume Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. See graphs, examples,. Air Pressure And Volume.
From www.criticalcarepractitioner.co.uk
Mechanical Ventilation Series Pressure/Volume loop….. Critical Care Air Pressure And Volume Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). In fact, if the volume. Explore the experiments and equations that describe these relationships and their applications. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Learn. Air Pressure And Volume.
From www.youtube.com
When air expands adiabatically its pressure p and volume v are related Air Pressure And Volume Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and. Air Pressure And Volume.
From derangedphysiology.com
Pressurevolume loops in the presence of lung pathology Deranged Air Pressure And Volume Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. See graphs, examples,. Air Pressure And Volume.
From www.criticalcarepractitioner.co.uk
Mechanical Ventilation Series Pressure/Volume loop….. Critical Care Air Pressure And Volume Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. In fact, if the volume. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume. Air Pressure And Volume.
From www.criticalcarepractitioner.co.uk
Mechanical Ventilation Series Pressure/Volume loop….. Critical Care Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Learn about the boyle's law. Air Pressure And Volume.
From www.pinterest.co.uk
A mathematical derivation of the equations relating the pressure Air Pressure And Volume Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. In fact, if the volume. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Learn how the volume and pressure of a gas are inversely proportional at constant temperature,. Air Pressure And Volume.
From derangedphysiology.com
Interpreting the shape of the pressurevolume loop Deranged Physiology Air Pressure And Volume In fact, if the volume. Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. See graphs, examples, and applications of this law to breathing and gas laws. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Decreasing the volume of a contained. Air Pressure And Volume.
From www.omniamfg.com
Understanding the PressureVolume Diagrams — Omnia MFG Air Pressure And Volume In fact, if the volume. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law.. Air Pressure And Volume.
From www.slideserve.com
PPT Gases PowerPoint Presentation, free download ID2195347 Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Boyle’s law relates the pressure and volume of a gas at constant temperature and amount. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Learn how the volume and pressure of a gas are inversely proportional at. Air Pressure And Volume.
From www.tes.com
GCSE AQA Physics P6.7 Pressure and Volume Teaching Resources Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Explore the experiments and equations. Air Pressure And Volume.
From www.youtube.com
PressureVolume Loops Compliance Respiratory Physiology YouTube Air Pressure And Volume Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Boyle’s. Air Pressure And Volume.
From www.nesscopressure.com.au
What is air pressure? Nessco Pressure Systems Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. In fact, if the volume. Learn how. Air Pressure And Volume.
From www.respiratorytherapyzone.com
Ventilator Waveforms and Graphics Made EASY (Overview) Air Pressure And Volume Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. In fact, if the volume. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional. Air Pressure And Volume.
From www.evomart.co.uk
Vol.5 Fundamentals Part 5 Compressors Evomart Air Pressure And Volume Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. In fact, if the volume. See graphs, examples, and applications of this law to breathing and gas laws. Learn how. Air Pressure And Volume.
From byjus.com
7. The volume of an air bubble is doubled as it rises o from the bottom Air Pressure And Volume See graphs, examples, and applications of this law to breathing and gas laws. Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. Explore the experiments and equations that describe these relationships and their applications. Learn how the volume and pressure of a gas are inversely proportional at constant. Air Pressure And Volume.
From courses.lumenlearning.com
Relating Pressure, Volume, Amount, and Temperature The Ideal Gas Law Air Pressure And Volume Boyle's law is a relation between pressure and volume of a gas at constant temperature, formulated by robert boyle in 1662. In fact, if the volume. Explore the experiments and equations that describe these relationships and their applications. Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Boyle’s law relates. Air Pressure And Volume.
From www.edplace.com
Understand Gas Pressure Worksheet EdPlace Air Pressure And Volume Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). See graphs, examples, and applications of this law to breathing and gas laws. Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Explore the experiments and equations. Air Pressure And Volume.
From aeromodelbasic.blogspot.com
model aircraft THE RELATIONS BETWEEN PRESSURE, VOLUME AND TEMPERATURE Air Pressure And Volume Learn how to derive it from kinetic theory, how real gases deviate from it, and see examples and applications. Learn about the boyle's law formula, which states that pressure and volume are inversely proportional to each other at constant temperature. Learn how the volume and pressure of a gas are inversely proportional at constant temperature, according to boyle’s law. Boyle's. Air Pressure And Volume.
From owlcation.com
Lung Pressures and Lung Compliance Owlcation Air Pressure And Volume In fact, if the volume. Learn how pressure and volume of a gas are inversely proportional (boyle's law), and how temperature and volume are directly proportional (charles's law). Decreasing the volume of a contained gas will increase its pressure, and increasing its volume will decrease its pressure. Learn about the boyle's law formula, which states that pressure and volume are. Air Pressure And Volume.