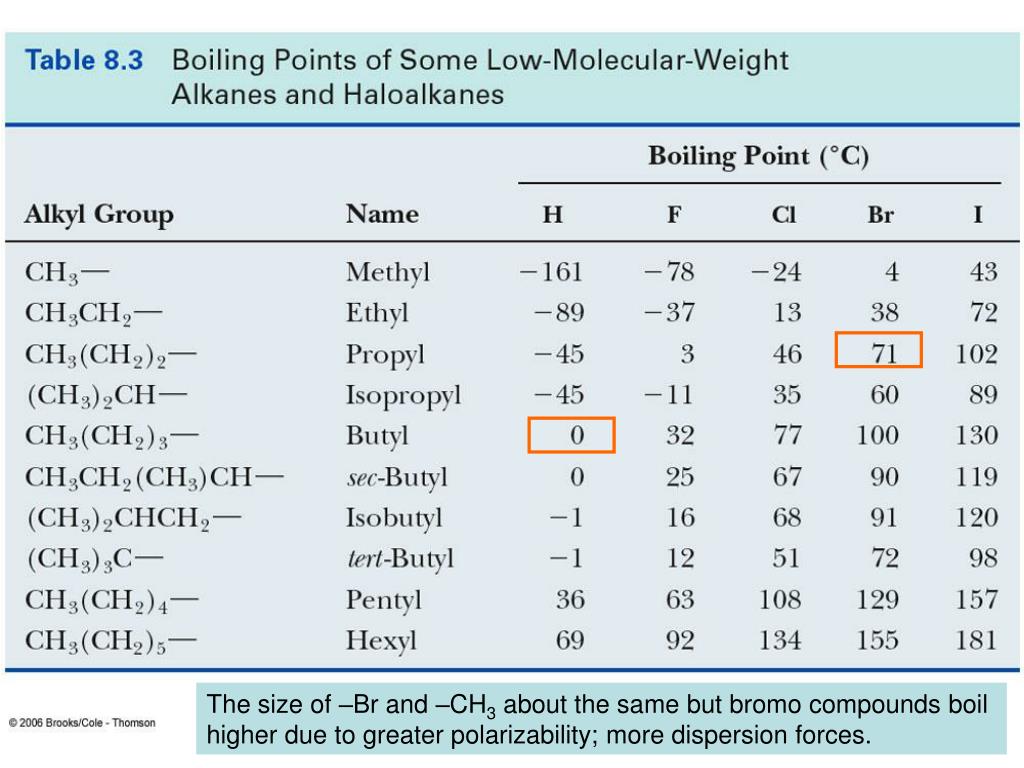
Boiling Point Of Bromine Fluoride . Pale brown (because of br 2) appearance: Fluorine has the lowest melting and boiling points. Fluorine, chlorine, bromine and iodine. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. The melting and boiling points then increase as you go down the group. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. You will see that both melting points and boiling points rise as you go down the group. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): A covalent bond is formed by the orbital. This includes their melting points, boiling. Elemental fluorine was first discovered in 1886 by isolating it from.
from www.slideserve.com
Fluorine, chlorine, bromine and iodine. Fluorine has the lowest melting and boiling points. Elemental fluorine was first discovered in 1886 by isolating it from. The melting and boiling points then increase as you go down the group. This includes their melting points, boiling. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): A covalent bond is formed by the orbital. Pale brown (because of br 2) appearance: You will see that both melting points and boiling points rise as you go down the group.
PPT Alkyl Halides PowerPoint Presentation, free download ID6686122
Boiling Point Of Bromine Fluoride It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): You will see that both melting points and boiling points rise as you go down the group. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. The melting and boiling points then increase as you go down the group. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Pale brown (because of br 2) appearance: This includes their melting points, boiling. Fluorine has the lowest melting and boiling points. A covalent bond is formed by the orbital. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. Fluorine, chlorine, bromine and iodine. Elemental fluorine was first discovered in 1886 by isolating it from.
From labbyag.es
Melting And Boiling Points Of Compounds Chart Labb by AG Boiling Point Of Bromine Fluoride This includes their melting points, boiling. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. You will see that both. Boiling Point Of Bromine Fluoride.
From www.toppr.com
Match the column I with Column II. Boiling Point Of Bromine Fluoride If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. Elemental fluorine was first discovered in 1886 by isolating it from. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. You will see that both melting points and boiling. Boiling Point Of Bromine Fluoride.
From www.numerade.com
SOLVED List the intermolecular forces present in hydrogen halides Boiling Point Of Bromine Fluoride A covalent bond is formed by the orbital. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Elemental fluorine was first discovered in 1886 by isolating it from. Fluorine has the lowest melting and. Boiling Point Of Bromine Fluoride.
From hxeyjyser.blob.core.windows.net
Bromine Element Melting And Boiling Point at Amber Charles blog Boiling Point Of Bromine Fluoride Fluorine, chlorine, bromine and iodine. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. This includes their melting points, boiling. Elemental fluorine was first discovered in 1886 by isolating it from. This page discusses the trends in the atomic and physical properties of the group 7. Boiling Point Of Bromine Fluoride.
From www.numerade.com
SOLVED List the intermolecular forces present in hydrogen halides Boiling Point Of Bromine Fluoride Fluorine, chlorine, bromine and iodine. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): A covalent bond is formed by the orbital. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. The melting and. Boiling Point Of Bromine Fluoride.
From www.gauthmath.com
Solved The boiling points of diatomic halogens are compared in the Boiling Point Of Bromine Fluoride Elemental fluorine was first discovered in 1886 by isolating it from. Pale brown (because of br 2) appearance: This includes their melting points, boiling. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. Fluorine has the lowest melting and boiling points. The melting and boiling points. Boiling Point Of Bromine Fluoride.
From slideplayer.com
Trends in Periodic Table ppt download Boiling Point Of Bromine Fluoride The melting and boiling points then increase as you go down the group. This includes their melting points, boiling. Fluorine, chlorine, bromine and iodine. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. The melting and boiling points of the group 7 elements increase going down the group which indicates that the. Boiling Point Of Bromine Fluoride.
From socratic.org
The liquefied hydrogen halides have the normal boiling points given Boiling Point Of Bromine Fluoride A covalent bond is formed by the orbital. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. This includes their melting points, boiling. Pale brown (because of br 2) appearance: Fluorine has the lowest melting and boiling points. The melting and boiling points then increase as you go down the group. You. Boiling Point Of Bromine Fluoride.
From www.numerade.com
SOLVED QUESTION The boiling points of the halogens are fluorine 188Â Boiling Point Of Bromine Fluoride You will see that both melting points and boiling points rise as you go down the group. The melting and boiling points then increase as you go down the group. This includes their melting points, boiling. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine. Boiling Point Of Bromine Fluoride.
From www.slideserve.com
PPT Alkyl Halides PowerPoint Presentation, free download ID6686122 Boiling Point Of Bromine Fluoride Fluorine, chlorine, bromine and iodine. Fluorine has the lowest melting and boiling points. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Elemental fluorine was first discovered in 1886 by isolating it from. This page discusses the trends in the atomic and physical. Boiling Point Of Bromine Fluoride.
From hxeyjyser.blob.core.windows.net
Bromine Element Melting And Boiling Point at Amber Charles blog Boiling Point Of Bromine Fluoride Fluorine has the lowest melting and boiling points. Fluorine, chlorine, bromine and iodine. Pale brown (because of br 2) appearance: This includes their melting points, boiling. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): A covalent bond is formed by the orbital. It can be seen that there is a. Boiling Point Of Bromine Fluoride.
From www.chemistrysteps.com
Boiling Point and Melting Point in Organic Chemistry Chemistry Steps Boiling Point Of Bromine Fluoride You will see that both melting points and boiling points rise as you go down the group. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Elemental fluorine was first discovered in 1886 by isolating it from. This page discusses the trends in. Boiling Point Of Bromine Fluoride.
From www.youtube.com
n butyl bromide has higher boiling point than isobutyl bromide YouTube Boiling Point Of Bromine Fluoride A covalent bond is formed by the orbital. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. Elemental fluorine was. Boiling Point Of Bromine Fluoride.
From www.chegg.com
Solved We have seen that the SN2 reaction of ethyl bromide Boiling Point Of Bromine Fluoride Elemental fluorine was first discovered in 1886 by isolating it from. Pale brown (because of br 2) appearance: The melting and boiling points then increase as you go down the group. A covalent bond is formed by the orbital. Fluorine has the lowest melting and boiling points. The melting and boiling points of the group 7 elements increase going down. Boiling Point Of Bromine Fluoride.
From www.numerade.com
SOLVED The picture below represents the particle of a compound The Boiling Point Of Bromine Fluoride The melting and boiling points then increase as you go down the group. A covalent bond is formed by the orbital. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. Elemental fluorine was first discovered in 1886 by isolating it from. Pale brown (because of br 2) appearance: This includes their melting. Boiling Point Of Bromine Fluoride.
From www.chegg.com
Solved The boiling point of bromine (Br2) is 332 K. What are Boiling Point Of Bromine Fluoride If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. This includes their melting points, boiling. Fluorine has the lowest melting and boiling points. A covalent bond is formed by the orbital. Fluorine, chlorine, bromine and iodine. The melting and boiling points of the group 7 elements increase going down the group which. Boiling Point Of Bromine Fluoride.
From slideplayer.com
THE HALOGENS. ppt download Boiling Point Of Bromine Fluoride This includes their melting points, boiling. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. Pale brown (because of br 2) appearance: The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. Fluorine, chlorine, bromine and iodine. A covalent. Boiling Point Of Bromine Fluoride.
From www.numerade.com
SOLVED What is the boiling point of bromine when the external pressure Boiling Point Of Bromine Fluoride If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. The melting and boiling points then increase as you go down the group. Fluorine, chlorine, bromine and. Boiling Point Of Bromine Fluoride.
From www.slideserve.com
PPT What do we know about fluorine, chlorine and bromine? PowerPoint Boiling Point Of Bromine Fluoride This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Elemental fluorine was first discovered in 1886 by isolating it from. A covalent bond is formed by the orbital. This includes their melting points, boiling. Pale brown (because of br 2) appearance: The melting and boiling points then increase as you go. Boiling Point Of Bromine Fluoride.
From www.nuclear-power.com
Bromine Melting Point Boiling Point Boiling Point Of Bromine Fluoride Pale brown (because of br 2) appearance: If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. You will see that both melting points and boiling points rise as you go down the group. Fluorine has the lowest melting and boiling points. This includes their melting points, boiling. The melting and boiling points. Boiling Point Of Bromine Fluoride.
From sciencing.com
Why Does the Boiling Point Increase When the Atomic Radius Increases in Boiling Point Of Bromine Fluoride Fluorine has the lowest melting and boiling points. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. This includes their melting points, boiling. It can be seen that. Boiling Point Of Bromine Fluoride.
From wisc.pb.unizin.org
Melting and Boiling Point Comparisons (M10Q2) UWMadison Chemistry Boiling Point Of Bromine Fluoride This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): The melting and boiling points then increase as you go down the group. Fluorine has the lowest melting and boiling points. Fluorine, chlorine, bromine and iodine. Pale brown (because of br 2) appearance: The melting and boiling points of the group 7. Boiling Point Of Bromine Fluoride.
From favpng.com
Hydrogen Bromide Hydrogen Fluoride Hydrochloric Acid Boiling Point, PNG Boiling Point Of Bromine Fluoride Pale brown (because of br 2) appearance: It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. This includes their melting points, boiling. Fluorine, chlorine, bromine and iodine. You will see that both melting points and boiling points rise as you go down the. Boiling Point Of Bromine Fluoride.
From slideplayer.com
Intermolecular forces ppt download Boiling Point Of Bromine Fluoride Elemental fluorine was first discovered in 1886 by isolating it from. The melting and boiling points then increase as you go down the group. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. A covalent bond is formed by the orbital. You will see that both. Boiling Point Of Bromine Fluoride.
From slideplayer.com
No Comp Book Question Write down your homework ppt download Boiling Point Of Bromine Fluoride A covalent bond is formed by the orbital. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. Pale brown (because of br 2) appearance: It can be seen that there is a regular increase. Boiling Point Of Bromine Fluoride.
From hxefdxmpv.blob.core.windows.net
Bromine Higher Boiling Point at Nina Colegrove blog Boiling Point Of Bromine Fluoride Pale brown (because of br 2) appearance: Fluorine has the lowest melting and boiling points. This includes their melting points, boiling. You will see that both melting points and boiling points rise as you go down the group. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): A covalent bond is. Boiling Point Of Bromine Fluoride.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID3665787 Boiling Point Of Bromine Fluoride You will see that both melting points and boiling points rise as you go down the group. Elemental fluorine was first discovered in 1886 by isolating it from. A covalent bond is formed by the orbital. This includes their melting points, boiling. Pale brown (because of br 2) appearance: The melting and boiling points then increase as you go down. Boiling Point Of Bromine Fluoride.
From www.shutterstock.com
Bromine Parodic Table Element Boiling Melting Stock Vector (Royalty Boiling Point Of Bromine Fluoride The melting and boiling points then increase as you go down the group. This includes their melting points, boiling. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Pale brown (because of br 2) appearance: Fluorine has the lowest melting and boiling points.. Boiling Point Of Bromine Fluoride.
From www.chegg.com
Solved The normal boiling point of bromine is 58.8∘C. Given Boiling Point Of Bromine Fluoride This includes their melting points, boiling. The melting and boiling points of the group 7 elements increase going down the group which indicates that the elements become less volatile. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. It can be seen that there is a regular increase in many of the. Boiling Point Of Bromine Fluoride.
From www.chegg.com
Solved The normal boiling point (at P=1 bar) of bromine Boiling Point Of Bromine Fluoride If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. It can be seen that there is a regular increase in many of the properties of the halogens proceeding down group 17 from fluorine to iodine. Pale brown (because of br 2) appearance: Fluorine, chlorine, bromine and iodine. This page discusses the trends. Boiling Point Of Bromine Fluoride.
From material-properties.org
Bromine Thermal Properties Melting Point Thermal Conductivity Boiling Point Of Bromine Fluoride You will see that both melting points and boiling points rise as you go down the group. This includes their melting points, boiling. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Pale brown (because of br 2) appearance: Fluorine, chlorine, bromine and iodine. Elemental fluorine was first discovered in 1886. Boiling Point Of Bromine Fluoride.
From www.numerade.com
SOLVED Rank the following compounds from lowest to highest boiling Boiling Point Of Bromine Fluoride Elemental fluorine was first discovered in 1886 by isolating it from. This includes their melting points, boiling. Fluorine, chlorine, bromine and iodine. A covalent bond is formed by the orbital. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. You will see that both melting points and boiling points rise as you. Boiling Point Of Bromine Fluoride.
From www.numerade.com
SOLVED 3. Look up the boiling points of methyl fluoride, methyl Boiling Point Of Bromine Fluoride Pale brown (because of br 2) appearance: This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Elemental fluorine was first discovered in 1886 by isolating it from. Fluorine has the lowest melting and boiling points. The melting and boiling points of the group 7 elements increase going down the group which. Boiling Point Of Bromine Fluoride.
From www.toppr.com
What will be the boiling point of bromine when 174.5mg of octa atomic Boiling Point Of Bromine Fluoride This includes their melting points, boiling. Fluorine, chlorine, bromine and iodine. This page discusses the trends in the atomic and physical properties of the group 7 elements (the halogens): Pale brown (because of br 2) appearance: A covalent bond is formed by the orbital. If you explore the graphs, you will find that fluorine and chlorine are gases at room. Boiling Point Of Bromine Fluoride.
From www.pinterest.com
Fluorine Halogens Chemistry, Element chemistry, Electron configuration Boiling Point Of Bromine Fluoride Elemental fluorine was first discovered in 1886 by isolating it from. If you explore the graphs, you will find that fluorine and chlorine are gases at room temperature,. Pale brown (because of br 2) appearance: The melting and boiling points then increase as you go down the group. The melting and boiling points of the group 7 elements increase going. Boiling Point Of Bromine Fluoride.