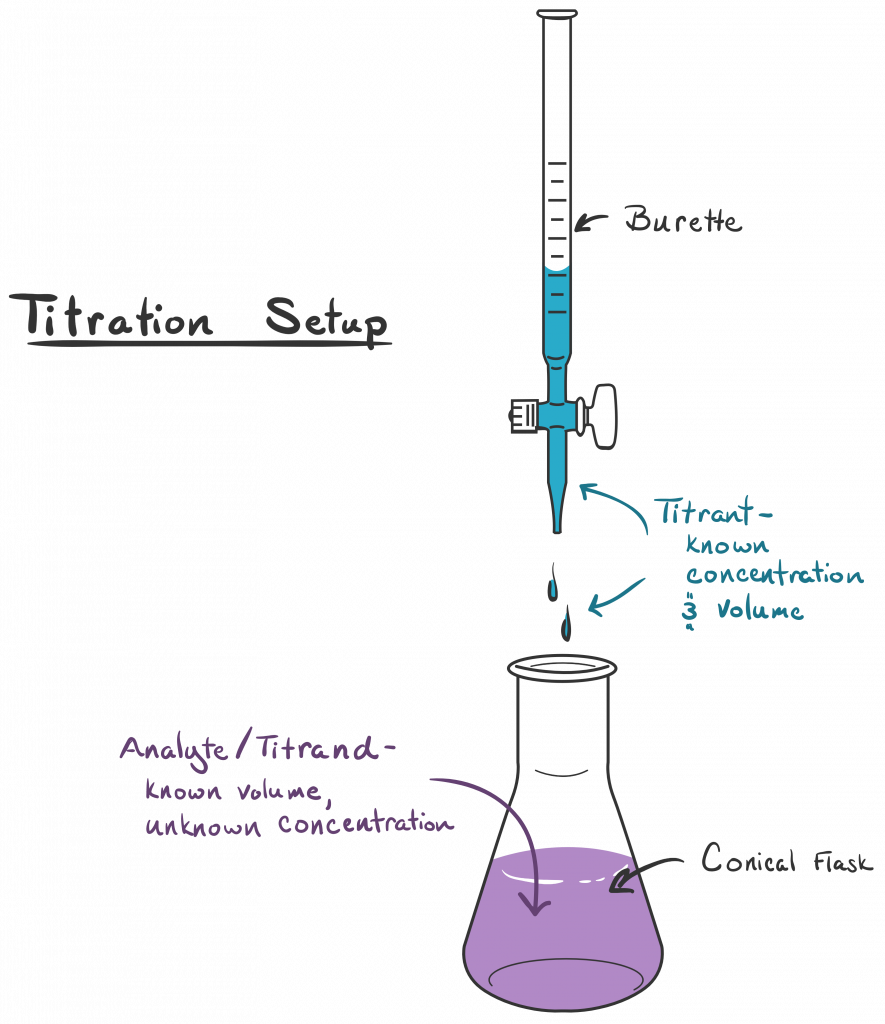
Figure Of Titration . (b) a known volume of sample is reacted with the titrant until (c) the ph. Sorting out some confusing terms. (a) titrant volume = 0 ml. Calculate the ph at these volumes of added base solution: Our titration calculator will help you never have to ask how do i calculate titrations? again. The equivalence point of a titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). What is the equivalence point of a titration. Learn the titration graph and titration equation. What are titrant and analyte. The solution ph is due to the acid ionization of hcl. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is.
from www.microlit.com
Our titration calculator will help you never have to ask how do i calculate titrations? again. Learn the titration graph and titration equation. What is the equivalence point of a titration. The equivalence point of a titration. What are titrant and analyte. The solution ph is due to the acid ionization of hcl. (a) titrant volume = 0 ml. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Calculate the ph at these volumes of added base solution:
An Advanced Guide to Titration Microlit
Figure Of Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. What is the equivalence point of a titration. The solution ph is due to the acid ionization of hcl. Our titration calculator will help you never have to ask how do i calculate titrations? again. Calculate the ph at these volumes of added base solution: Sorting out some confusing terms. What are titrant and analyte. The equivalence point of a titration. (b) a known volume of sample is reacted with the titrant until (c) the ph. (a) titrant volume = 0 ml. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Learn the titration graph and titration equation.
From chemistrylabs-2.blogspot.com
Titration Apparatus Diagram Chemistry Labs Figure Of Titration What is the equivalence point of a titration. Our titration calculator will help you never have to ask how do i calculate titrations? again. Calculate the ph at these volumes of added base solution: Learn the titration graph and titration equation. The equivalence point of a titration. (b) a known volume of sample is reacted with the titrant until (c). Figure Of Titration.
From www.vecteezy.com
Acid base titration experiment and phases of color change during Figure Of Titration What is the equivalence point of a titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). The solution ph is due to the acid ionization of hcl. Our titration calculator will help you never have to ask how do. Figure Of Titration.
From www.researchgate.net
Potentiometric titration curves and first derivates of native and Figure Of Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is.. Figure Of Titration.
From www.slideserve.com
PPT Ch 6 Precipitation Titrations, Sec 65 and 66 PowerPoint Figure Of Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Sorting out some confusing terms. The solution ph is due to the acid ionization of hcl. A titration is a volumetric technique in which a solution of one reactant (the titrant). Figure Of Titration.
From www.researchgate.net
Complexometric (FeEDTA) titration for determination of iron in water Figure Of Titration Sorting out some confusing terms. The solution ph is due to the acid ionization of hcl. The equivalence point of a titration. (b) a known volume of sample is reacted with the titrant until (c) the ph. What are titrant and analyte. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to. Figure Of Titration.
From hubpages.com
Different Methods of Measuring Drug Potency, Concentration, Efficacy Figure Of Titration Learn the titration graph and titration equation. What are titrant and analyte. Sorting out some confusing terms. (a) titrant volume = 0 ml. Calculate the ph at these volumes of added base solution: (b) a known volume of sample is reacted with the titrant until (c) the ph. What is the equivalence point of a titration. The solution ph is. Figure Of Titration.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Figure Of Titration The solution ph is due to the acid ionization of hcl. Sorting out some confusing terms. Calculate the ph at these volumes of added base solution: A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). (b) a known volume of. Figure Of Titration.
From www.youtube.com
How to Do Titration Calculations // HSC Chemistry YouTube Figure Of Titration What is the equivalence point of a titration. Sorting out some confusing terms. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. The solution ph is due to the acid ionization of hcl. A titration is carried out. Figure Of Titration.
From general.chemistrysteps.com
Titration of a Weak Acid by a Strong Base Chemistry Steps Figure Of Titration What is the equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Learn the titration graph and titration equation. Calculate the ph at these volumes of added base solution: What are titrant and. Figure Of Titration.
From mungfali.com
Acid Base Titration Calculation Figure Of Titration The equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Sorting out some confusing terms. Learn the titration graph and titration equation. The solution ph is due to the acid ionization of hcl.. Figure Of Titration.
From aydin-well-mahoney.blogspot.com
What Best Describes the Purpose of a Titration Figure Of Titration Sorting out some confusing terms. What is the equivalence point of a titration. The solution ph is due to the acid ionization of hcl. Calculate the ph at these volumes of added base solution: A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte). Figure Of Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Figure Of Titration Sorting out some confusing terms. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). What is the equivalence point of a titration. The solution ph is due to the acid ionization of hcl. Calculate the ph at these volumes of. Figure Of Titration.
From chemwiki.ucdavis.edu
Titration of a Weak Base with a Strong Acid Chemwiki Figure Of Titration What are titrant and analyte. Learn the titration graph and titration equation. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). The equivalence point of a titration. Sorting out some confusing terms. A titration is a volumetric technique in which. Figure Of Titration.
From scienceready.com.au
Titration Standard Solution, Washing, Setup HSC Chemistry Science Figure Of Titration Sorting out some confusing terms. Learn the titration graph and titration equation. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Calculate the ph at these volumes of added base solution: What is the equivalence point of a titration. (b). Figure Of Titration.
From mmerevise.co.uk
Titrations and Uncertainties MME Figure Of Titration A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. What is the equivalence point of a titration. Calculate the ph at these volumes of added base solution: (b) a known volume of sample is reacted with the titrant. Figure Of Titration.
From bramblechemistry.weebly.com
4C6 Titration Figure Of Titration The equivalence point of a titration. Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Learn the titration graph and titration equation.. Figure Of Titration.
From app.jove.com
AcidBase/ pH Titration Curves and Equivalence Points Concept Figure Of Titration Learn the titration graph and titration equation. Sorting out some confusing terms. What is the equivalence point of a titration. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). (a) titrant volume = 0 ml. Calculate the ph at these. Figure Of Titration.
From www.researchgate.net
The schematic diagram of titration equipment to titrate the composite Figure Of Titration Our titration calculator will help you never have to ask how do i calculate titrations? again. What is the equivalence point of a titration. The solution ph is due to the acid ionization of hcl. Sorting out some confusing terms. Calculate the ph at these volumes of added base solution: (b) a known volume of sample is reacted with the. Figure Of Titration.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Figure Of Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is.. Figure Of Titration.
From byjus.com
Titration of Oxalic Acid with KMnO4 Chemistry Practicals Class 12 Figure Of Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Calculate the ph at these volumes of added base solution: Sorting out some confusing terms. What are titrant and analyte. The solution ph is due to the acid ionization of hcl.. Figure Of Titration.
From wisc.pb.unizin.org
M16Q4 Titration of a Strong Acid with a Strong Base Chem 103/104 Figure Of Titration What is the equivalence point of a titration. Sorting out some confusing terms. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). The solution ph is due to the acid ionization of hcl. (a) titrant volume = 0 ml. A. Figure Of Titration.
From www.chemicals.co.uk
What is Titration in Chemistry? The Chemistry Blog Figure Of Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Sorting out some confusing terms. Calculate the ph at these volumes of added base solution: What are titrant and analyte. (a) titrant volume = 0 ml. The equivalence point of a. Figure Of Titration.
From www.microlit.com
An Advanced Guide to Titration Microlit Figure Of Titration (b) a known volume of sample is reacted with the titrant until (c) the ph. Calculate the ph at these volumes of added base solution: What are titrant and analyte. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). A. Figure Of Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Figure Of Titration (b) a known volume of sample is reacted with the titrant until (c) the ph. The equivalence point of a titration. (a) titrant volume = 0 ml. What is the equivalence point of a titration. What are titrant and analyte. Learn the titration graph and titration equation. A titration is carried out for 25.00 ml of 0.100 m hcl (strong. Figure Of Titration.
From chem.libretexts.org
9.1 Overview of Titrimetry Chemistry LibreTexts Figure Of Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). (a) titrant volume = 0 ml. Calculate the ph at these volumes of added base solution: Sorting out some confusing terms. The solution ph is due to the acid ionization of. Figure Of Titration.
From theedge.com.hk
Chemistry How To Titration The Edge Figure Of Titration What is the equivalence point of a titration. (a) titrant volume = 0 ml. The solution ph is due to the acid ionization of hcl. (b) a known volume of sample is reacted with the titrant until (c) the ph. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution. Figure Of Titration.
From chem.libretexts.org
9.2 AcidBase Titrations Chemistry LibreTexts Figure Of Titration Learn the titration graph and titration equation. The equivalence point of a titration. (a) titrant volume = 0 ml. What is the equivalence point of a titration. Sorting out some confusing terms. Our titration calculator will help you never have to ask how do i calculate titrations? again. (b) a known volume of sample is reacted with the titrant until. Figure Of Titration.
From general.chemistrysteps.com
Titration of a Weak Base by a Strong Acid Chemistry Steps Figure Of Titration Calculate the ph at these volumes of added base solution: Our titration calculator will help you never have to ask how do i calculate titrations? again. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. What are titrant. Figure Of Titration.
From courses.lumenlearning.com
AcidBase Titrations Chemistry Figure Of Titration Learn the titration graph and titration equation. What is the equivalence point of a titration. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. (a) titrant volume = 0 ml. Sorting out some confusing terms. What are titrant. Figure Of Titration.
From chemistrymadesimple.net
What is Titration and How is it Done? Chemistry Made Simple Figure Of Titration The solution ph is due to the acid ionization of hcl. Our titration calculator will help you never have to ask how do i calculate titrations? again. (a) titrant volume = 0 ml. What is the equivalence point of a titration. Calculate the ph at these volumes of added base solution: Sorting out some confusing terms. A titration is a. Figure Of Titration.
From www.researchgate.net
Representative example of conductometric titration from CNC batch 3 Figure Of Titration What are titrant and analyte. Calculate the ph at these volumes of added base solution: The solution ph is due to the acid ionization of hcl. (b) a known volume of sample is reacted with the titrant until (c) the ph. Learn the titration graph and titration equation. (a) titrant volume = 0 ml. A titration is a volumetric technique. Figure Of Titration.
From www.youtube.com
Acid Base Titration Problems, Basic Introduction, Calculations Figure Of Titration (b) a known volume of sample is reacted with the titrant until (c) the ph. What are titrant and analyte. A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). Calculate the ph at these volumes of added base solution: Learn. Figure Of Titration.
From www.chemistryscl.com
Titrimetry, Titration Classifications, Standard solutions, Equivalence Figure Of Titration Calculate the ph at these volumes of added base solution: Sorting out some confusing terms. What are titrant and analyte. (a) titrant volume = 0 ml. Learn the titration graph and titration equation. Our titration calculator will help you never have to ask how do i calculate titrations? again. The solution ph is due to the acid ionization of hcl.. Figure Of Titration.
From ar.inspiredpencil.com
Titration Setup Diagram Figure Of Titration A titration is carried out for 25.00 ml of 0.100 m hcl (strong acid) with 0.100 m of a strong base naoh (the titration curve is shown in [link]). (a) titrant volume = 0 ml. (b) a known volume of sample is reacted with the titrant until (c) the ph. Calculate the ph at these volumes of added base solution:. Figure Of Titration.
From letitsnowglobe.co.uk
Titration procedure pdf Figure Of Titration The solution ph is due to the acid ionization of hcl. A titration is a volumetric technique in which a solution of one reactant (the titrant) is added to a solution of a second reactant (the analyte) until the equivalence point is. Calculate the ph at these volumes of added base solution: What is the equivalence point of a titration.. Figure Of Titration.