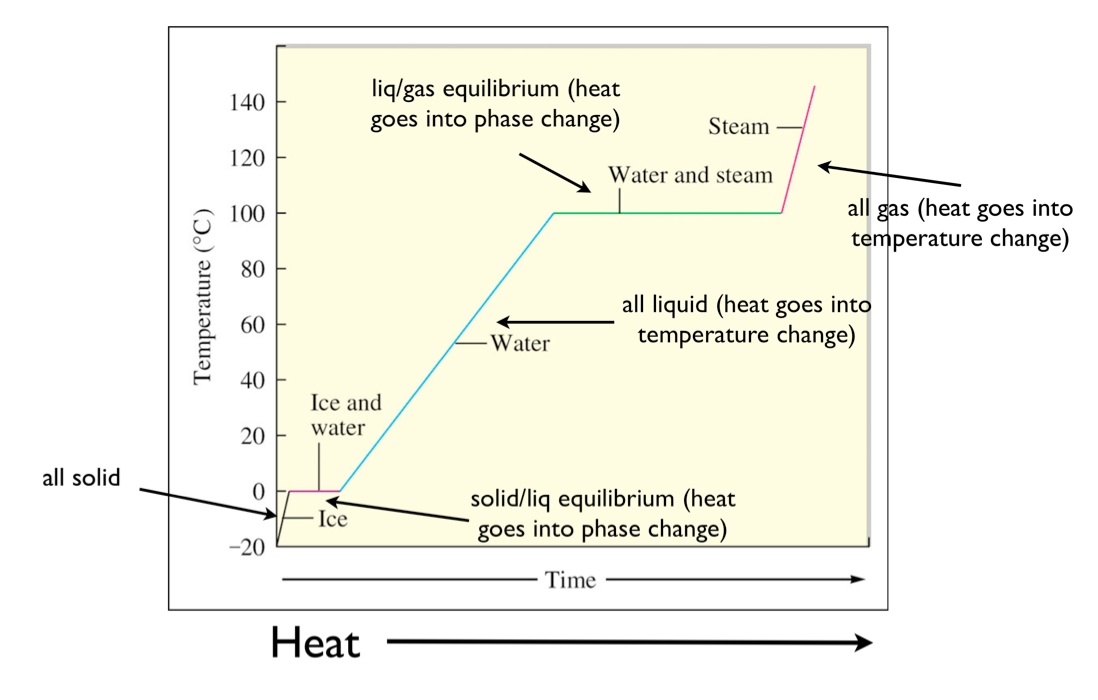
Heating Curves Energy . Heating curves show how the temperature changes as a substance is heated up. Cooling curves are the opposite. Phase diagrams (plots of pressure vs. These two types of plots. The ice is in a closed container. As heat is steadily added to the. Temperature) were correlated with heating curves (plots of temperature vs. Look through the slide show below to find out more about. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. They show how the temperature changes as a substance is. A heating curve for water.
from ch301.cm.utexas.edu
Cooling curves are the opposite. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. Look through the slide show below to find out more about. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Heating curves show how the temperature changes as a substance is heated up. The ice is in a closed container. A heating curve for water. Phase diagrams (plots of pressure vs. As heat is steadily added to the. These two types of plots.
heating curve
Heating Curves Energy Phase diagrams (plots of pressure vs. Look through the slide show below to find out more about. Temperature) were correlated with heating curves (plots of temperature vs. Phase diagrams (plots of pressure vs. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Cooling curves are the opposite. A heating curve for water. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. As heat is steadily added to the. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. They show how the temperature changes as a substance is. These two types of plots. The ice is in a closed container. Heating curves show how the temperature changes as a substance is heated up.
From www.slideserve.com
PPT OB Practice phase concepts cooling and heating curves, phase Heating Curves Energy Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. These two types of plots. As heat is steadily added to the. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the.. Heating Curves Energy.
From studyschoolburman.z21.web.core.windows.net
What Is A Heat Curve Heating Curves Energy Phase diagrams (plots of pressure vs. Look through the slide show below to find out more about. These two types of plots. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. As heat is steadily added to the. A heating curve for water. A heating. Heating Curves Energy.
From www.worldwisetutoring.com
Heating and Cooling Curves Heating Curves Energy These two types of plots. Heating curves show how the temperature changes as a substance is heated up. A heating curve for water. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. They show how the temperature changes as a substance is. A heating curve. Heating Curves Energy.
From www.youtube.com
HEATING CURVE How to Read & How TO Draw A Heating Curve [ AboodyTV Heating Curves Energy The ice is in a closed container. Cooling curves are the opposite. These two types of plots. Temperature) were correlated with heating curves (plots of temperature vs. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. They show how the temperature changes as a substance. Heating Curves Energy.
From slideplayer.com
Heating Curves and Phase Diagrams ppt download Heating Curves Energy They show how the temperature changes as a substance is. Heating curves show how the temperature changes as a substance is heated up. The ice is in a closed container. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. Phase diagrams (plots of pressure vs. The amount of energy needed. Heating Curves Energy.
From www.slideserve.com
PPT Thermochemistry The heat energy of chemical reactions PowerPoint Heating Curves Energy The ice is in a closed container. Phase diagrams (plots of pressure vs. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. These two types of plots. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o. Heating Curves Energy.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free Heating Curves Energy Look through the slide show below to find out more about. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. Heating. Heating Curves Energy.
From materialdbhutchins.z21.web.core.windows.net
What Are Heating And Cooling Curves Heating Curves Energy As heat is steadily added to the. The ice is in a closed container. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Phase diagrams (plots of pressure vs. A heating curve for water. Heating curves show how the temperature changes as a substance is. Heating Curves Energy.
From preparatorychemistry.com
Heating Curve Heating Curves Energy A heating curve for water. Cooling curves are the opposite. Phase diagrams (plots of pressure vs. These two types of plots. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. Heating curves show how the temperature changes as a substance is heated up. Imagine that. Heating Curves Energy.
From www.youtube.com
Heating Curves and Energy Calculations YouTube Heating Curves Energy As heat is steadily added to the. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. Cooling curves are the opposite. They show how the temperature changes as a substance is. Phase diagrams (plots of pressure vs. The ice is in a closed container. Temperature). Heating Curves Energy.
From tech-controllers.com
Heating curve what is it and how to set it? TECH Controllers Heating Curves Energy Phase diagrams (plots of pressure vs. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. A heating curve for water. Look through the slide show below to find out more about. A heating curve is a plot of temperature versus time that represents the phase. Heating Curves Energy.
From socratic.org
What are the 6 phase changes along a heating curve? Socratic Heating Curves Energy These two types of plots. The ice is in a closed container. A heating curve for water. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. They show how the temperature changes as a substance is. Cooling curves are the opposite. As heat is steadily. Heating Curves Energy.
From www.youtube.com
AP Video 10.6 Intro to HeatingCooling Curves & Calculations YouTube Heating Curves Energy Temperature) were correlated with heating curves (plots of temperature vs. Cooling curves are the opposite. These two types of plots. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. As heat is steadily added to the. They show how the temperature changes as a substance is. Look through the slide. Heating Curves Energy.
From chem-net.blogspot.com
Phase Changes Energy Changes Heating Curves Chemistry Net Heating Curves Energy The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. Cooling curves are the opposite. They show how the temperature changes as a substance is. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below. Heating Curves Energy.
From chem.libretexts.org
8.1 Heating Curves and Phase Changes Chemistry LibreTexts Heating Curves Energy Look through the slide show below to find out more about. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Phase diagrams (plots of pressure vs. The ice is in a closed container. As heat is steadily added to the. Cooling curves are the opposite.. Heating Curves Energy.
From wisc.pb.unizin.org
Heating Curves and Phase Diagrams (M11Q2) UWMadison Chemistry 103/ Heating Curves Energy Heating curves show how the temperature changes as a substance is heated up. Phase diagrams (plots of pressure vs. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. Look through the slide show below to find out more about. The amount of energy needed to sublime 1 mol of a. Heating Curves Energy.
From www.youtube.com
Heating and Cooling Curve / Introduction plus and Potential Heating Curves Energy Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. As heat is steadily added to the. Temperature) were correlated with heating curves (plots of temperature vs.. Heating Curves Energy.
From app.jove.com
Heating and Cooling Curves Concept Chemistry JoVe Heating Curves Energy A heating curve for water. Look through the slide show below to find out more about. They show how the temperature changes as a substance is. Phase diagrams (plots of pressure vs. These two types of plots. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. Imagine that you have. Heating Curves Energy.
From www.slideserve.com
PPT Phase Diagrams & Heating Curves PowerPoint Presentation, free Heating Curves Energy Look through the slide show below to find out more about. The ice is in a closed container. Phase diagrams (plots of pressure vs. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. A heating curve is a plot of temperature versus time that represents. Heating Curves Energy.
From www.youtube.com
Heating Curves Temperature Energy Graphs GCSE Physics YouTube Heating Curves Energy Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Cooling curves are the opposite. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. The ice is in a closed container. As heat is steadily added. Heating Curves Energy.
From www.slideserve.com
PPT Heat, Energy and Phases of Matter PowerPoint Presentation, free Heating Curves Energy These two types of plots. As heat is steadily added to the. Look through the slide show below to find out more about. Cooling curves are the opposite. Temperature) were correlated with heating curves (plots of temperature vs. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. They show how. Heating Curves Energy.
From www.slideserve.com
PPT Thermal Energy PowerPoint Presentation, free download ID5077760 Heating Curves Energy A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. They show how the temperature changes as a substance is. A heating curve for water. Temperature) were correlated with heating curves (plots of temperature vs. Look through the slide show below to find out more about. Phase diagrams (plots of pressure. Heating Curves Energy.
From www.slideserve.com
PPT Heating/Cooling Curves & Q= mC Δ T PowerPoint Presentation ID Heating Curves Energy Temperature) were correlated with heating curves (plots of temperature vs. A heating curve for water. Phase diagrams (plots of pressure vs. Cooling curves are the opposite. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. As heat is steadily added to the. The amount of energy needed to sublime 1. Heating Curves Energy.
From www.chegg.com
Solved The graph above shows the heating curve of water. One Heating Curves Energy Look through the slide show below to find out more about. Temperature) were correlated with heating curves (plots of temperature vs. A heating curve for water. Heating curves show how the temperature changes as a substance is heated up. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. As heat. Heating Curves Energy.
From www.slideserve.com
PPT Heating and Cooling Curves PowerPoint Presentation, free download Heating Curves Energy These two types of plots. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. A heating curve for water. Cooling curves are the opposite. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. They show. Heating Curves Energy.
From www.ck12.org
Heating and Cooling Curves ( Read ) Chemistry CK12 Foundation Heating Curves Energy A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. As heat is steadily added to the. A heating curve for water. Look through the slide show. Heating Curves Energy.
From www.npro.energy
Heating curve for building energy systems nPro Heating Curves Energy Look through the slide show below to find out more about. The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. Heating curves show how the temperature changes as a substance is heated up. Temperature) were correlated with heating curves (plots of temperature vs. Imagine that. Heating Curves Energy.
From study.com
Heating & Cooling Curves Definition, Phases & Examples Lesson Heating Curves Energy Cooling curves are the opposite. Temperature) were correlated with heating curves (plots of temperature vs. Phase diagrams (plots of pressure vs. The ice is in a closed container. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. As heat is steadily added to the. They show how the temperature changes. Heating Curves Energy.
From www.doubtnut.com
The heating curve of a particular substance in solid state is a shown Heating Curves Energy They show how the temperature changes as a substance is. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. The ice is in a closed container. These two types of plots. As heat is steadily added to the. Heating curves show how the temperature changes as a substance is heated. Heating Curves Energy.
From slideplayer.com
Heating and Cooling Curves ppt download Heating Curves Energy Phase diagrams (plots of pressure vs. Heating curves show how the temperature changes as a substance is heated up. Look through the slide show below to find out more about. They show how the temperature changes as a substance is. The ice is in a closed container. As heat is steadily added to the. A heating curve is a plot. Heating Curves Energy.
From study.com
Phase Changes and Heating Curves Lesson Heating Curves Energy Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. Temperature) were correlated with heating curves (plots of temperature vs. They show how the temperature changes as a substance is. The ice is in a closed container. A heating curve for water. Look through the slide. Heating Curves Energy.
From slideplayer.com
Introduction to Thermochemistry ppt download Heating Curves Energy Cooling curves are the opposite. They show how the temperature changes as a substance is. A heating curve for water. As heat is steadily added to the. The ice is in a closed container. These two types of plots. A heating curve is a plot of temperature versus time that represents the phase changes that a substance undergoes. The amount. Heating Curves Energy.
From www.slideserve.com
PPT Heating/Cooling Curves & Q= mC Δ T PowerPoint Presentation ID Heating Curves Energy As heat is steadily added to the. Phase diagrams (plots of pressure vs. They show how the temperature changes as a substance is. The ice is in a closed container. Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The amount of energy needed to. Heating Curves Energy.
From ch301.cm.utexas.edu
heating curve Heating Curves Energy The amount of energy needed to sublime 1 mol of a substance is its enthalpy of sublimation (δhsub) and is the sum of the. They show how the temperature changes as a substance is. These two types of plots. The ice is in a closed container. Heating curves show how the temperature changes as a substance is heated up. Cooling. Heating Curves Energy.
From www.expii.com
Heating and Cooling Curves — Overview & Examples Expii Heating Curves Energy Imagine that you have a block of ice that is at a temperature of −30oc − 30 o c, well below its melting point. The ice is in a closed container. As heat is steadily added to the. Phase diagrams (plots of pressure vs. They show how the temperature changes as a substance is. Cooling curves are the opposite. Heating. Heating Curves Energy.