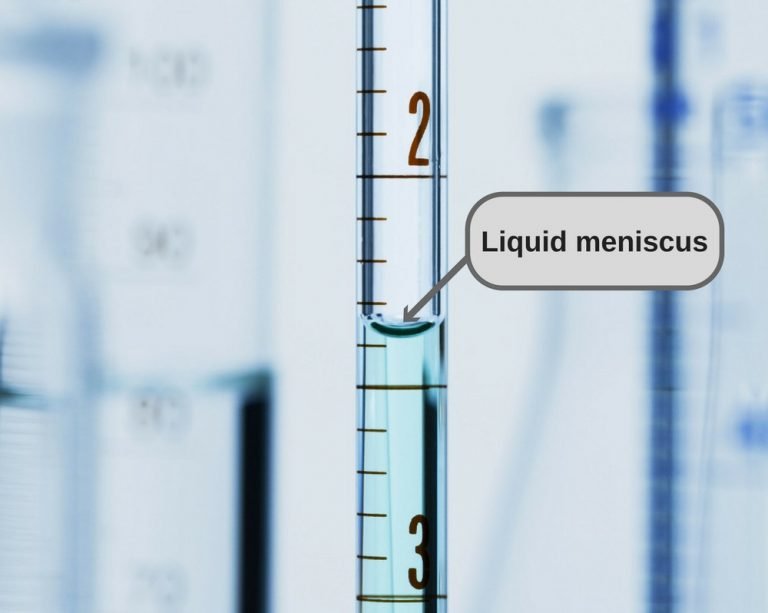
Why Does Water Stick To Itself . These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. Because of hydrogen bonding, water can actually support objects that are more dense than it is. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Water is sticky and clumps together into drops because of its cohesive. Water is transported in plants through both cohesive and adhesive forces; Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Cohesion is the ability of water to “stick” to itself. Cohesion holds hydrogen bonds together to create surface tension on water. Water molecules stick to one another on the. Since water is attracted to other molecules, adhesive forces pull the. The hydrogen bonding between water molecules means they are more likely to. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity.
from www.scienceabc.com
Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Water is transported in plants through both cohesive and adhesive forces; These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Cohesion is the ability of water to “stick” to itself. Water molecules stick to one another on the. Water is sticky and clumps together into drops because of its cohesive. These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. Because of hydrogen bonding, water can actually support objects that are more dense than it is. Cohesion holds hydrogen bonds together to create surface tension on water.
Water Pouring Why Does Water Stick To Glass When Pouring?
Why Does Water Stick To Itself Water is sticky and clumps together into drops because of its cohesive. Cohesion is the ability of water to “stick” to itself. The hydrogen bonding between water molecules means they are more likely to. Water molecules stick to one another on the. Water is transported in plants through both cohesive and adhesive forces; In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Since water is attracted to other molecules, adhesive forces pull the. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Water is sticky and clumps together into drops because of its cohesive. Cohesion holds hydrogen bonds together to create surface tension on water. Because of hydrogen bonding, water can actually support objects that are more dense than it is.
From www.scienceabc.com
Water Pouring Why Does Water Stick To Glass When Pouring? Why Does Water Stick To Itself Cohesion is the ability of water to “stick” to itself. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. Water is transported in plants through both cohesive. Why Does Water Stick To Itself.
From www.sciencefacts.net
Water Expansion When Freezing Why Does Water Stick To Itself Water molecules stick to one another on the. Because of hydrogen bonding, water can actually support objects that are more dense than it is. Cohesion is the ability of water to “stick” to itself. Since water is attracted to other molecules, adhesive forces pull the. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules.. Why Does Water Stick To Itself.
From slideplayer.com
Properties of Water. ppt download Why Does Water Stick To Itself Water molecules stick to one another on the. Because of hydrogen bonding, water can actually support objects that are more dense than it is. These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. Since water is attracted to other molecules, adhesive forces pull the. Since water is attracted to. Why Does Water Stick To Itself.
From www.slideserve.com
PPT IT’S A WATER WATER WORLD PowerPoint Presentation, free download Why Does Water Stick To Itself Since water is attracted to other molecules, adhesive forces pull the. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. The hydrogen bonding between water molecules means they are more likely to.. Why Does Water Stick To Itself.
From www.expii.com
Cohesion and Adhesion (Water) — Properties & Examples Expii Why Does Water Stick To Itself Water molecules stick to one another on the. The hydrogen bonding between water molecules means they are more likely to. Because of hydrogen bonding, water can actually support objects that are more dense than it is. Cohesion is the ability of water to “stick” to itself. These forces pull water and the dissolved minerals from the roots to the leaves. Why Does Water Stick To Itself.
From slideplayer.com
Properties of Water 8/27/2015 Objective SWBAT observe the properties Why Does Water Stick To Itself These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Water molecules stick to one another on the. Cohesion is the ability of water to “stick” to itself. Cohesion holds hydrogen bonds together to create surface tension on water. These forces pull water and the dissolved minerals from the roots. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Why Does Water Stick To Itself Water is transported in plants through both cohesive and adhesive forces; Cohesion holds hydrogen bonds together to create surface tension on water. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. The hydrogen bonding between water molecules means they are more likely to. Water is sticky and. Why Does Water Stick To Itself.
From www.youtube.com
Why Does Water Stick to Your Cup When Pouring? (Coanda Effect) YouTube Why Does Water Stick To Itself In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Cohesion is the ability of water to “stick” to itself. Since water is attracted to other molecules, adhesive forces pull the. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. The. Why Does Water Stick To Itself.
From www.youtube.com
Why does water stick to your glass when pouring? YouTube Why Does Water Stick To Itself Because of hydrogen bonding, water can actually support objects that are more dense than it is. Water is sticky and clumps together into drops because of its cohesive. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. In water, each hydrogen nucleus is covalently bound to the central oxygen. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Understanding Water PowerPoint Presentation, free download ID Why Does Water Stick To Itself In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. The height to which capillary action will take water in a circular tube is limited by surface. Why Does Water Stick To Itself.
From www.youtube.com
Why does water stick together for kids? YouTube Why Does Water Stick To Itself Water is sticky and clumps together into drops because of its cohesive. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Since water is attracted to other molecules, adhesive forces pull the. Cohesion holds. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Water PowerPoint Presentation, free download ID2600281 Why Does Water Stick To Itself The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. Cohesion holds hydrogen bonds together to create surface tension on water. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. The hydrogen bonding between water molecules means they are more likely to.. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation, free download ID Why Does Water Stick To Itself In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Water is transported in plants through both cohesive and adhesive forces; These hydrogen bonds explain why water has a high boiling point,. Why Does Water Stick To Itself.
From www.scienceabc.com
Water Pouring Why Does Water Stick To Glass When Pouring? Why Does Water Stick To Itself Water molecules stick to one another on the. Cohesion holds hydrogen bonds together to create surface tension on water. The hydrogen bonding between water molecules means they are more likely to. Because of hydrogen bonding, water can actually support objects that are more dense than it is. Since water is attracted to other molecules, adhesive forces pull the water toward. Why Does Water Stick To Itself.
From www.usgs.gov
The strong polar bond between water molecules creates water cohesion. Why Does Water Stick To Itself Water molecules stick to one another on the. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. Because of hydrogen bonding, water can actually support objects that are more dense than it is. Cohesion. Why Does Water Stick To Itself.
From www.reddit.com
[ELI5] Why does water sometimes stick to glass when you pour it? r Why Does Water Stick To Itself These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Since water is attracted to other molecules, adhesive forces pull the. Since water is attracted to other molecules, adhesive forces. Why Does Water Stick To Itself.
From www.youtube.com
Usage of alkaline water stick YouTube Why Does Water Stick To Itself Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Water is transported in plants through both cohesive and adhesive forces; Because of hydrogen bonding, water can actually support objects that are more dense than it is. Water is sticky and clumps together into drops because of its cohesive. Cohesion holds hydrogen bonds together to. Why Does Water Stick To Itself.
From www.scienceabc.com
Water Pouring Why Does Water Stick To Glass When Pouring? Why Does Water Stick To Itself In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Water molecules stick to one another on the. Cohesion is the ability of water to “stick” to. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Notes Water and its Special Properties PowerPoint Presentation Why Does Water Stick To Itself The hydrogen bonding between water molecules means they are more likely to. Water is sticky and clumps together into drops because of its cohesive. Water molecules stick to one another on the. Water is transported in plants through both cohesive and adhesive forces; Because of hydrogen bonding, water can actually support objects that are more dense than it is. These. Why Does Water Stick To Itself.
From slideplayer.com
CHPT. 3 The Chemistry of Water Prof. Dr. Samih Tamimi. ppt download Why Does Water Stick To Itself Water molecules stick to one another on the. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Since water is attracted to other molecules,. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download Why Does Water Stick To Itself The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. Because of hydrogen bonding, water can actually support objects that are more dense than it is. These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. These hydrogen. Why Does Water Stick To Itself.
From www.scienceabc.com
Water Pouring Why Does Water Stick To Glass When Pouring? Why Does Water Stick To Itself The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. Because of hydrogen bonding, water can actually support objects that are more dense than it is. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Water is. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Earth’s Environmental Systems PowerPoint Presentation, free Why Does Water Stick To Itself Cohesion holds hydrogen bonds together to create surface tension on water. Because of hydrogen bonding, water can actually support objects that are more dense than it is. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. These hydrogen bonds explain why water has a high boiling point,. Why Does Water Stick To Itself.
From slideplayer.com
Water The elixir of life!!. ppt download Why Does Water Stick To Itself Since water is attracted to other molecules, adhesive forces pull the. Because of hydrogen bonding, water can actually support objects that are more dense than it is. Water is transported in plants through both cohesive and adhesive forces; These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Since water. Why Does Water Stick To Itself.
From bio.libretexts.org
4.5.1.3 CohesionTension Theory Biology LibreTexts Why Does Water Stick To Itself These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other surfaces (adhesion),. Since water is attracted to other molecules, adhesive forces pull the. Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by. Why Does Water Stick To Itself.
From courses.lumenlearning.com
Water Potential Biology I Why Does Water Stick To Itself These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. These hydrogen bonds explain why water has a high boiling point, sticks to itself (cohesion), sticks to other. Why Does Water Stick To Itself.
From www.youtube.com
Why Does Water Stick to Glass When I Pour It? YouTube Why Does Water Stick To Itself In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The height to which capillary action will take water in a circular tube is limited by surface tension and, of course, gravity. The hydrogen bonding between water molecules means they are more likely to. Because of hydrogen. Why Does Water Stick To Itself.
From slideplayer.com
Basic Chemical Bonding and Properties of Water ppt download Why Does Water Stick To Itself Cohesion holds hydrogen bonds together to create surface tension on water. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Since water is attracted to other molecules, adhesive forces pull the. The hydrogen bonding between water molecules means they are more likely to. These hydrogen bonds. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Horticultural Responses to Water PowerPoint Presentation, free Why Does Water Stick To Itself Because of hydrogen bonding, water can actually support objects that are more dense than it is. These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. The hydrogen bonding between water molecules means they are more likely to. In water, each hydrogen nucleus is covalently bound to the central oxygen. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Chapter 6 and 7 PowerPoint Presentation, free download ID1957632 Why Does Water Stick To Itself Cohesion holds hydrogen bonds together to create surface tension on water. Because of hydrogen bonding, water can actually support objects that are more dense than it is. Water is transported in plants through both cohesive and adhesive forces; Water molecules stick to one another on the. The hydrogen bonding between water molecules means they are more likely to. Since water. Why Does Water Stick To Itself.
From www.scienceabc.com
Water Pouring Why Does Water Stick To Glass When Pouring? Why Does Water Stick To Itself These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. Water is transported in plants through both cohesive and adhesive forces; Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Water molecules stick to one another on the. Water is sticky and clumps together. Why Does Water Stick To Itself.
From www.youtube.com
Why does water boil in a vacuum? YouTube Why Does Water Stick To Itself Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Cohesion holds hydrogen bonds together to create surface tension on water. Water is sticky and clumps together into drops because of its cohesive. Since water is attracted to other molecules, adhesive forces pull the. Water is transported in plants through both cohesive and adhesive forces;. Why Does Water Stick To Itself.
From www.slideserve.com
PPT Properties of Water PowerPoint Presentation ID1783102 Why Does Water Stick To Itself Since water is attracted to other molecules, adhesive forces pull the water toward other molecules. Because of hydrogen bonding, water can actually support objects that are more dense than it is. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. Water is transported in plants through. Why Does Water Stick To Itself.
From brainly.com
Water is essential to life on Earth its unique physical and chemical Why Does Water Stick To Itself Cohesion holds hydrogen bonds together to create surface tension on water. Water molecules stick to one another on the. In water, each hydrogen nucleus is covalently bound to the central oxygen atom by a pair of electrons that are shared between them. The height to which capillary action will take water in a circular tube is limited by surface tension. Why Does Water Stick To Itself.
From slideplayer.com
Properties of Water. ppt download Why Does Water Stick To Itself These forces pull water and the dissolved minerals from the roots to the leaves and other parts of the plant. Water is sticky and clumps together into drops because of its cohesive. Cohesion holds hydrogen bonds together to create surface tension on water. The height to which capillary action will take water in a circular tube is limited by surface. Why Does Water Stick To Itself.