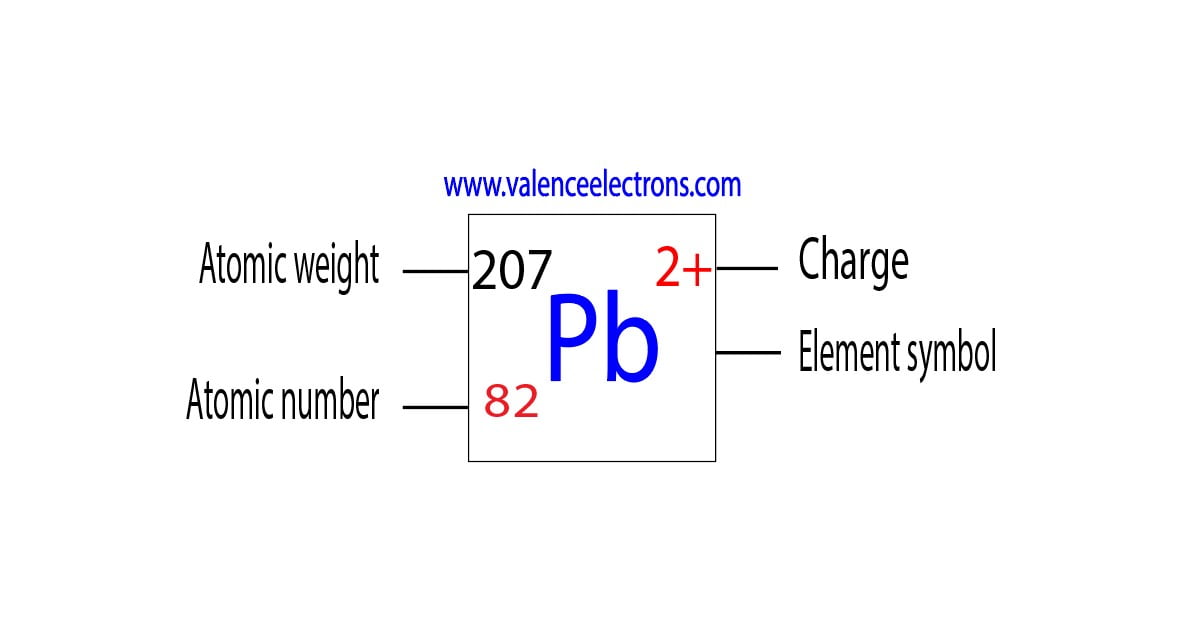
Lead To Electrons . View rotating bohr models for all 118 elements. Atomic mass, electron configurations, charges, and more. 119 rows access detailed info on all elements: Write out the full configuration for lead as follows: The shorthand for lead makes use of the configuration of xenon,. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Lead has an atomic number z = 82, and so it has 82 electrons.
from valenceelectrons.com
Atomic mass, electron configurations, charges, and more. Write out the full configuration for lead as follows: The shorthand for lead makes use of the configuration of xenon,. 119 rows access detailed info on all elements: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. View rotating bohr models for all 118 elements. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. Lead has an atomic number z = 82, and so it has 82 electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making.
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+)
Lead To Electrons The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. View rotating bohr models for all 118 elements. Lead has an atomic number z = 82, and so it has 82 electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. Write out the full configuration for lead as follows: The shorthand for lead makes use of the configuration of xenon,. Atomic mass, electron configurations, charges, and more. 119 rows access detailed info on all elements: A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making.
From valenceelectrons.com
Lead(Pb) Electron Configuration and Orbital Diagram Lead To Electrons A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. View rotating bohr models for all 118 elements. Lead has an atomic number z = 82, and so it has 82 electrons. 119 rows access detailed info on all elements: Write out. Lead To Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead To Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. View rotating bohr models for all 118 elements. Lead has an. Lead To Electrons.
From valenceelectrons.com
Electron Configuration for Lead and Lead ions(Pb2+, Pb4+) Lead To Electrons Atomic mass, electron configurations, charges, and more. Lead has an atomic number z = 82, and so it has 82 electrons. 119 rows access detailed info on all elements: The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6. Lead To Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead To Electrons The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. View rotating bohr models for all 118 elements. 93 rows you may. Lead To Electrons.
From www.thoughtco.com
Atoms Diagrams Electron Configurations of Elements Lead To Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Write out the full configuration for lead as follows: A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. The lead. Lead To Electrons.
From www.sciencephoto.com
Lead, atomic structure Stock Image C045/6429 Science Photo Library Lead To Electrons The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. A strong metallic bond will be the result of more delocalized electrons,. Lead To Electrons.
From www.nuclear-power.com
Lead Atomic Number Atomic Mass Density of Lead Lead To Electrons A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s. Lead To Electrons.
From periodictable.me
Valency of Lead Archives Dynamic Periodic Table of Elements and Chemistry Lead To Electrons 119 rows access detailed info on all elements: The shorthand for lead makes use of the configuration of xenon,. Lead has an atomic number z = 82, and so it has 82 electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect. Lead To Electrons.
From exovwrlic.blob.core.windows.net
Pb Lead Valence Electrons at Eve Dehart blog Lead To Electrons 119 rows access detailed info on all elements: A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p. Lead To Electrons.
From valenceelectrons.com
Protons, Neutrons, Electrons for Lead (Pb, Pb2+, Pb4+) Lead To Electrons 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2,. Lead To Electrons.
From material-properties.org
Lead Protons Neutrons Electrons Electron Configuration Lead To Electrons A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. 119 rows access detailed info on all elements: The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p. Lead To Electrons.
From iperiodictable.com
How To Find an Valence Lead Electron Configuration (Pb) Lead To Electrons Lead has an atomic number z = 82, and so it has 82 electrons. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom. Lead To Electrons.
From joifefnuf.blob.core.windows.net
Lead Complete Electron Configuration at Dawn Westbury blog Lead To Electrons Write out the full configuration for lead as follows: View rotating bohr models for all 118 elements. The shorthand for lead makes use of the configuration of xenon,. Lead has an atomic number z = 82, and so it has 82 electrons. 119 rows access detailed info on all elements: The lead electron configuration, represented as 6s 2 4f 14. Lead To Electrons.
From www.youtube.com
Electron Configuration of Lead Pb Lesson YouTube Lead To Electrons 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. Write out the full configuration for lead as follows: The shorthand for lead makes use of the configuration of xenon,. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The lead electron configuration,. Lead To Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead To Electrons 119 rows access detailed info on all elements: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. The lead electron. Lead To Electrons.
From awesomehome.co
Lead Periodic Table Electrons Awesome Home Lead To Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. View rotating bohr models for all 118 elements. 119 rows access detailed info on all elements: Atomic mass, electron configurations, charges, and more. Lead has an atomic number z = 82, and so it has 82 electrons. The shorthand. Lead To Electrons.
From www.schoolmykids.com
Compare Thallium vs Lead Periodic Table Element Comparison Compare Lead To Electrons Write out the full configuration for lead as follows: The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. Lead has an. Lead To Electrons.
From www.alamy.com
Atom symbol and electron of lead illustration Stock Vector Image & Art Lead To Electrons The shorthand for lead makes use of the configuration of xenon,. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. View. Lead To Electrons.
From www.youtube.com
Electron Configuration for Pb, Pb2+, and Pb4+ (Lead and Lead Ions Lead To Electrons Atomic mass, electron configurations, charges, and more. Write out the full configuration for lead as follows: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. 119 rows access detailed info on all elements: A strong metallic bond will be the result of more delocalized electrons, which causes the. Lead To Electrons.
From derekcarrsavvy-chemist.blogspot.com
savvychemist Ionic Bonding (2) Dot and cross diagrams/Lewis structures Lead To Electrons Write out the full configuration for lead as follows: View rotating bohr models for all 118 elements. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10. Lead To Electrons.
From www.sciencephoto.com
Lead, atomic structure Stock Image C013/1639 Science Photo Library Lead To Electrons View rotating bohr models for all 118 elements. Atomic mass, electron configurations, charges, and more. The shorthand for lead makes use of the configuration of xenon,. Write out the full configuration for lead as follows: 119 rows access detailed info on all elements: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an. Lead To Electrons.
From awesomehome.co
Lead Periodic Table Electrons Awesome Home Lead To Electrons Lead has an atomic number z = 82, and so it has 82 electrons. 119 rows access detailed info on all elements: The shorthand for lead makes use of the configuration of xenon,. Atomic mass, electron configurations, charges, and more. View rotating bohr models for all 118 elements. 93 rows you may assume the valences of the chemical elements—the number. Lead To Electrons.
From dxovcaane.blob.core.windows.net
Shorthand Electron Configuration For Lead at Perry blog Lead To Electrons The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. 119 rows access detailed info on all elements: Lead has an atomic. Lead To Electrons.
From www.alamy.com
Pb Lead, Periodic Table of the Elements, Shell Structure of Lead Lead To Electrons View rotating bohr models for all 118 elements. Write out the full configuration for lead as follows: Lead has an atomic number z = 82, and so it has 82 electrons. The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10. Lead To Electrons.
From www.schoolmykids.com
Lead (Pb) Element Information, Facts, Properties, Uses Periodic Lead To Electrons A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. Write out the full configuration for lead as follows: 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Lead has. Lead To Electrons.
From cabinet.matttroy.net
Lead Periodic Table Protons Neutrons And Electrons Matttroy Lead To Electrons The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. Atomic mass, electron configurations, charges, and more. Write out the full configuration. Lead To Electrons.
From quizlet.com
The diagram below shows some subatomic particles. A diagram, Quizlet Lead To Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Lead has an atomic number z = 82, and so it has 82 electrons. The shorthand for lead makes use of the configuration of xenon,. Write out the full configuration for lead as follows: 119 rows access detailed info. Lead To Electrons.
From valenceelectrons.com
Complete Electron Configuration for Lead (Pb, Pb2+, Pb4+) Lead To Electrons 119 rows access detailed info on all elements: A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. Atomic mass, electron configurations, charges, and more. Write out the full configuration for lead as follows: The lead electron configuration, represented as 6s 2. Lead To Electrons.
From www.slideserve.com
PPT Electron Dot Structures PowerPoint Presentation, free download Lead To Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. The shorthand for lead makes use of the configuration of xenon,. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making.. Lead To Electrons.
From www.dreamstime.com
Diagram Representation of the Element Lead Stock Vector Illustration Lead To Electrons A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. Write out the full configuration for lead as follows: View rotating bohr models for all 118 elements. 93 rows you may assume the valences of the chemical elements—the number of electrons with. Lead To Electrons.
From courses.lumenlearning.com
Electrons Biology for Majors I Lead To Electrons The shorthand for lead makes use of the configuration of xenon,. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or.. Lead To Electrons.
From www.youtube.com
CHEMISTRY 101 Writing an Electron Configuration for Lead Using the Lead To Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making. Lead has an atomic number z = 82, and so it. Lead To Electrons.
From www.alamy.com
Lead atom Cut Out Stock Images & Pictures Alamy Lead To Electrons The shorthand for lead makes use of the configuration of xenon,. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. A strong metallic bond will be the result of more delocalized electrons, which causes the effective nuclear charge on electrons on the cation to increase, in effect making.. Lead To Electrons.
From www.youtube.com
How to find Protons & Electrons for the Pb2+ and Pb4+ (Lead II and Lead Lead To Electrons The lead electron configuration, represented as 6s 2 4f 14 5d 10 6p 2 or 1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 2, illustrates the precise. Lead has an atomic number z = 82, and so it has. Lead To Electrons.
From ar.inspiredpencil.com
Lead Atom Electrons Lead To Electrons 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or. Lead has an atomic number z = 82, and so it has 82 electrons. 119 rows access detailed info on all elements: The shorthand for lead makes use of the configuration of xenon,. The lead electron configuration, represented as. Lead To Electrons.