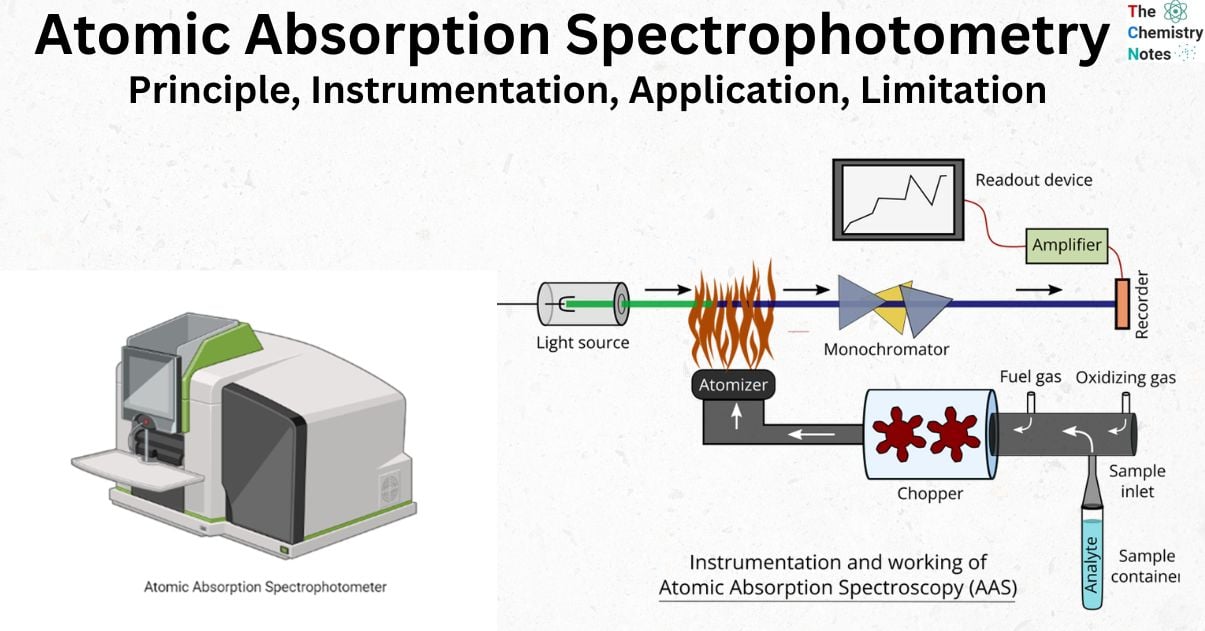
Atomic Absorption Spectroscopy With Example . Guystav kirchoff and robert bunsen first used atomic absorption. A(λ) = ε(λ)bc = log po/p. Atomic emission spectroscopy measures the. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. In aas, the flame functions as (i) sample holder, (ii) desolvation.
from scienceinfo.com
A(λ) = ε(λ)bc = log po/p. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Guystav kirchoff and robert bunsen first used atomic absorption. Atomic emission spectroscopy measures the. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In aas, the flame functions as (i) sample holder, (ii) desolvation. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples.
Atomic Absorption Spectrophotometry Principle, Parts, Uses
Atomic Absorption Spectroscopy With Example Guystav kirchoff and robert bunsen first used atomic absorption. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Guystav kirchoff and robert bunsen first used atomic absorption. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Atomic emission spectroscopy measures the. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. A(λ) = ε(λ)bc = log po/p. In aas, the flame functions as (i) sample holder, (ii) desolvation.
From science.williams.edu
Atomic Absorption Spectrometer (AAS) Science Center Atomic Absorption Spectroscopy With Example Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. Atomic emission spectroscopy measures the. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the. Atomic Absorption Spectroscopy With Example.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy (AAS)I PowerPoint Presentation Atomic Absorption Spectroscopy With Example In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Guystav kirchoff and robert bunsen first used atomic absorption. A(λ) = ε(λ)bc = log po/p. The amount. Atomic Absorption Spectroscopy With Example.
From namrataheda.blogspot.com
B for Biology Spectrophotometry Atomic Absorption Spectrophotometry Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder. Atomic Absorption Spectroscopy With Example.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy (AAS)I PowerPoint Presentation Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In aas, the flame functions as (i) sample holder, (ii) desolvation. Guystav kirchoff and robert bunsen first used atomic absorption. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the. Atomic Absorption Spectroscopy With Example.
From www.azolifesciences.com
Atomic Absorption Spectroscopy (AAS) An Overview Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In aas, the flame functions as (i) sample holder, (ii) desolvation. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined. Atomic Absorption Spectroscopy With Example.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy PowerPoint Presentation, free Atomic Absorption Spectroscopy With Example Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In aas, the flame functions as (i) sample holder, (ii) desolvation. Guystav kirchoff and robert bunsen first used atomic absorption. A(λ) = ε(λ)bc. Atomic Absorption Spectroscopy With Example.
From www.researchgate.net
(PDF) Atomic Absorption Spectroscopy (AAS) Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. A(λ) = ε(λ)bc = log po/p. Both atomic emission and atomic absorption spectroscopy can be used. Atomic Absorption Spectroscopy With Example.
From www.slideshare.net
atomic absorption spectroscopy Atomic Absorption Spectroscopy With Example In aas, the flame functions as (i) sample holder, (ii) desolvation. Guystav kirchoff and robert bunsen first used atomic absorption. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder. Atomic Absorption Spectroscopy With Example.
From www.pharmacy.cuhk.edu.hk
lab2Atomic Absorption Spectrophotometer (AAS) (ID 1.6) School of Atomic Absorption Spectroscopy With Example Guystav kirchoff and robert bunsen first used atomic absorption. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In aas, the flame functions as (i) sample holder, (ii) desolvation. A(λ) = ε(λ)bc = log po/p. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. Atomic. Atomic Absorption Spectroscopy With Example.
From www.slideshare.net
Atomic absorption spectroscopy Atomic Absorption Spectroscopy With Example Atomic emission spectroscopy measures the. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Guystav kirchoff and robert bunsen first used atomic absorption. In aas, the flame functions as (i) sample holder, (ii) desolvation. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. Atomic absorption. Atomic Absorption Spectroscopy With Example.
From www.slideshare.net
Atomic absorption spectroscopy Atomic Absorption Spectroscopy With Example In aas, the flame functions as (i) sample holder, (ii) desolvation. Guystav kirchoff and robert bunsen first used atomic absorption. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. Atomic emission spectroscopy measures the. The amount of light absorbed at this wavelength is directly proportional. Atomic Absorption Spectroscopy With Example.
From www.youtube.com
Introduction to Atomic Spectroscopy YouTube Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder. Atomic Absorption Spectroscopy With Example.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Atomic Absorption Spectroscopy With Example Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. A(λ) = ε(λ)bc = log po/p. Atomic emission spectroscopy measures the. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light. Atomic Absorption Spectroscopy With Example.
From www.slideshare.net
ATOMIC ABSORPTION SPECTROSCOPY Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder. Atomic Absorption Spectroscopy With Example.
From www.youtube.com
Atomic Absorption Spectroscopy/Atomic Absorption Spectrometry/AAS YouTube Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder. Atomic Absorption Spectroscopy With Example.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy (AAS)I PowerPoint Presentation Atomic Absorption Spectroscopy With Example Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In aas, the flame functions as (i) sample holder, (ii) desolvation. Guystav kirchoff and robert bunsen first used atomic absorption. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. A(λ) = ε(λ)bc = log po/p. In. Atomic Absorption Spectroscopy With Example.
From www.slideshare.net
Atomic Spectroscopy Basic Principles and Instruments Atomic Absorption Spectroscopy With Example Atomic emission spectroscopy measures the. In aas, the flame functions as (i) sample holder, (ii) desolvation. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. A(λ) = ε(λ)bc = log po/p. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In atomic absorption spectroscopy, the. Atomic Absorption Spectroscopy With Example.
From users.highland.edu
Atomic Spectra and Models of the Atom Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In aas, the flame functions as (i) sample holder, (ii) desolvation. A(λ) = ε(λ)bc = log po/p. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount. Atomic Absorption Spectroscopy With Example.
From www.youtube.com
Atomic Absorption Spectroscopy Introduction & instrumentation YouTube Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. A(λ) = ε(λ)bc = log po/p. Guystav kirchoff and robert bunsen first used atomic absorption. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In aas, the flame functions as (i) sample holder, (ii) desolvation. In. Atomic Absorption Spectroscopy With Example.
From www.studypool.com
SOLUTION Atomic Absorption Spectroscopy Studypool Atomic Absorption Spectroscopy With Example Atomic emission spectroscopy measures the. Guystav kirchoff and robert bunsen first used atomic absorption. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. A(λ) = ε(λ)bc. Atomic Absorption Spectroscopy With Example.
From www.youtube.com
Atomic Absorption Spectrometer (AAS) YouTube Atomic Absorption Spectroscopy With Example Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. Guystav kirchoff and robert bunsen first used atomic absorption. In aas, the flame functions as (i) sample holder, (ii) desolvation. Atomic emission spectroscopy measures the. Both atomic emission and atomic absorption spectroscopy can be used to. Atomic Absorption Spectroscopy With Example.
From www.biophlox.com
Buy Atomic Absorption Spectroscopy get price for lab equipment Atomic Absorption Spectroscopy With Example In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Atomic emission spectroscopy measures the. The amount of light absorbed at this wavelength is directly proportional to. Atomic Absorption Spectroscopy With Example.
From www.youtube.com
Atomic Absorption Spectroscopy AAS Forensic Science Instrumentation Atomic Absorption Spectroscopy With Example Atomic emission spectroscopy measures the. A(λ) = ε(λ)bc = log po/p. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. Atomic absorption spectrometry (aas) is a. Atomic Absorption Spectroscopy With Example.
From www.studypool.com
SOLUTION Atomic Absorption Spectroscopy Studypool Atomic Absorption Spectroscopy With Example Atomic emission spectroscopy measures the. In aas, the flame functions as (i) sample holder, (ii) desolvation. Guystav kirchoff and robert bunsen first used atomic absorption. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. A(λ) =. Atomic Absorption Spectroscopy With Example.
From www.pinterest.com
Absorption spectrum of the elements spectroscopy Physics, Earth and Atomic Absorption Spectroscopy With Example Guystav kirchoff and robert bunsen first used atomic absorption. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In aas, the flame functions as (i) sample holder, (ii) desolvation. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. A(λ) = ε(λ)bc = log po/p. Atomic. Atomic Absorption Spectroscopy With Example.
From blogs.maryville.edu
Sample Atomization Atomic Absorption Spectroscopy Learning Module Atomic Absorption Spectroscopy With Example Guystav kirchoff and robert bunsen first used atomic absorption. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. A(λ) = ε(λ)bc = log po/p. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. Both. Atomic Absorption Spectroscopy With Example.
From www.youtube.com
Atomic Absorption Spectroscopy YouTube Atomic Absorption Spectroscopy With Example In aas, the flame functions as (i) sample holder, (ii) desolvation. A(λ) = ε(λ)bc = log po/p. Atomic emission spectroscopy measures the. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. Guystav. Atomic Absorption Spectroscopy With Example.
From scienceinfo.com
Atomic Absorption Spectroscopy Instrumentation Atomic Absorption Spectroscopy With Example Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. In aas, the flame functions as (i) sample holder, (ii) desolvation. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In atomic absorption spectroscopy, the. Atomic Absorption Spectroscopy With Example.
From www.slideshare.net
Atomic absorption spectroscopy Atomic Absorption Spectroscopy With Example The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. In aas, the flame functions as (i) sample holder, (ii) desolvation. A(λ) = ε(λ)bc = log. Atomic Absorption Spectroscopy With Example.
From www.studocu.com
Chapter 2 Atomic Absorption spectroscopy Page 1 of 4 Objectives Atomic Absorption Spectroscopy With Example Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. In aas, the flame functions as (i) sample holder, (ii) desolvation. Guystav kirchoff and robert bunsen first used atomic absorption. A(λ) = ε(λ)bc = log po/p. In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and. Atomic Absorption Spectroscopy With Example.
From forensicfield.blog
Atomic Absorption Spectroscopy Forensic's blog Atomic Absorption Spectroscopy With Example Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. A(λ) = ε(λ)bc = log po/p. Atomic emission spectroscopy measures the. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. Both atomic emission and atomic. Atomic Absorption Spectroscopy With Example.
From scienceinfo.com
Atomic Absorption Spectrophotometry Principle, Parts, Uses Atomic Absorption Spectroscopy With Example In atomic absorption spectroscopy, the wavelength of absorbed light is determined by the type of atom (which element it is) and the amount of light is absorbed is determined as concentration of the element in the sample by the recorder and detector accordingly. In aas, the flame functions as (i) sample holder, (ii) desolvation. Atomic emission spectroscopy measures the. The. Atomic Absorption Spectroscopy With Example.
From www.youtube.com
Atomic Absorption Spectroscopy (AAS) Explained PART 1 YouTube Atomic Absorption Spectroscopy With Example Atomic emission spectroscopy measures the. Both atomic emission and atomic absorption spectroscopy can be used to analyze samples. Atomic absorption spectrometry (aas) is a technique for measuring quantities of chemical elements present in environmental samples by measuring the absorbed radiation by the. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions. Atomic Absorption Spectroscopy With Example.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy PowerPoint Presentation, free Atomic Absorption Spectroscopy With Example Guystav kirchoff and robert bunsen first used atomic absorption. Atomic emission spectroscopy measures the. In aas, the flame functions as (i) sample holder, (ii) desolvation. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. A(λ) = ε(λ)bc = log po/p. Both atomic emission and atomic absorption spectroscopy can be. Atomic Absorption Spectroscopy With Example.
From www.scribd.com
Aas Emission Spectrum Atomic Absorption Spectroscopy Atomic Absorption Spectroscopy With Example Guystav kirchoff and robert bunsen first used atomic absorption. Atomic emission spectroscopy measures the. The amount of light absorbed at this wavelength is directly proportional to the concentration of the absorbing ions or atoms. In aas, the flame functions as (i) sample holder, (ii) desolvation. A(λ) = ε(λ)bc = log po/p. Atomic absorption spectrometry (aas) is a technique for measuring. Atomic Absorption Spectroscopy With Example.