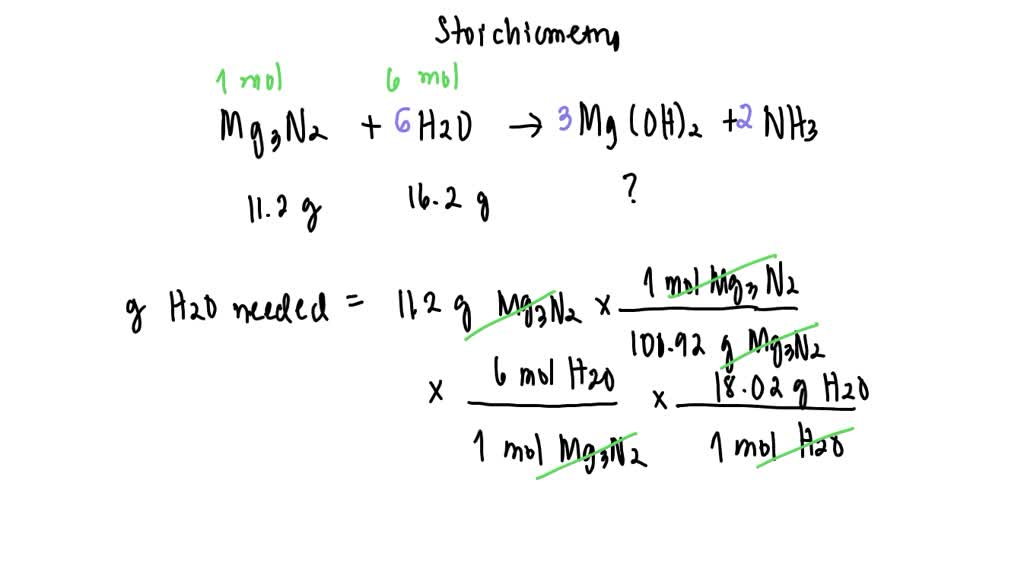
Magnesium Nitride + Water Equation . Magnesium nitride + water = magnesium hydroxide + ammonia. Or ammonia at 700 °c: Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. 3 mg + 2 nh3 → mg3n2 + 3. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. By passing dry nitrogen over heated magnesium at 800 °c: In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. 3 mg + n2 → mg3n2. One mole of magnesium nitride [mg 3 n 2] and six moles of. Magnesium nitride + water = ammonia + magnesium oxide. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide.
from www.numerade.com
Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. Or ammonia at 700 °c: 3 mg + 2 nh3 → mg3n2 + 3. By passing dry nitrogen over heated magnesium at 800 °c: For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. 3 mg + n2 → mg3n2. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. One mole of magnesium nitride [mg 3 n 2] and six moles of.
SOLVED For the following reaction, 12.1 grams of magnesium nitride are
Magnesium Nitride + Water Equation Magnesium nitride + water = magnesium hydroxide + ammonia. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. 3 mg + 2 nh3 → mg3n2 + 3. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. 3 mg + n2 → mg3n2. Magnesium nitride + water = magnesium hydroxide + ammonia. One mole of magnesium nitride [mg 3 n 2] and six moles of. Or ammonia at 700 °c: Magnesium nitride + water = ammonia + magnesium oxide. By passing dry nitrogen over heated magnesium at 800 °c:
From www.numerade.com
SOLVED Magnesium and nitrogen react in a combination reaction to Magnesium Nitride + Water Equation In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Magnesium nitride + water = magnesium hydroxide + ammonia. 3 mg + 2 nh3 → mg3n2 + 3. Magnesium nitride + water = ammonia + magnesium oxide. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. 3 mg. Magnesium Nitride + Water Equation.
From www.youtube.com
How to Balance Mg3N2 + H2O = MgO + NH3 (Magnesium nitride + Water Magnesium Nitride + Water Equation Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. One mole of magnesium nitride [mg 3 n 2] and six moles of. 3 mg + 2 nh3 → mg3n2 + 3. Magnesium nitride + water = magnesium hydroxide + ammonia. By passing dry nitrogen over. Magnesium Nitride + Water Equation.
From chrisnewslawrence.blogspot.com
What Is the Formula Mass of Magnesium Nitride Magnesium Nitride + Water Equation Magnesium nitride + water = magnesium hydroxide + ammonia. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. One mole of magnesium nitride [mg 3 n 2] and six moles of. Magnesium nitride + water = ammonia + magnesium oxide. 3 mg + n2 → mg3n2.. Magnesium Nitride + Water Equation.
From www.chegg.com
Solved A 6.81 g simple of magnesium nitride is reacted with Magnesium Nitride + Water Equation Or ammonia at 700 °c: By passing dry nitrogen over heated magnesium at 800 °c: Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. 3 mg + n2 → mg3n2. 3 mg + 2 nh3 → mg3n2 + 3. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. For example, we have mg_3n_2 on. Magnesium Nitride + Water Equation.
From www.shutterstock.com
Magnesium Nitride Formula Handwritten Chemical Formula Stock Magnesium Nitride + Water Equation In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Magnesium nitride + water = ammonia + magnesium oxide. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Or ammonia at 700 °c: 3 mg + n2 → mg3n2. By passing dry nitrogen over heated magnesium at 800. Magnesium Nitride + Water Equation.
From byjus.com
8. 24 gm pure sample of magnesium is burned in air to form magnesium Magnesium Nitride + Water Equation Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. By passing dry nitrogen over heated magnesium at 800 °c: Magnesium nitride + water = ammonia + magnesium oxide. One mole of magnesium nitride [mg 3. Magnesium Nitride + Water Equation.
From www.youtube.com
Type of Reaction for Mg + N2 = Mg3N2 YouTube Magnesium Nitride + Water Equation Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. By passing dry nitrogen over heated magnesium at 800 °c: Or ammonia at 700 °c: Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. One mole of magnesium nitride [mg 3 n 2] and. Magnesium Nitride + Water Equation.
From chrisnewslawrence.blogspot.com
What Is the Formula Mass of Magnesium Nitride Magnesium Nitride + Water Equation One mole of magnesium nitride [mg 3 n 2] and six moles of. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. By passing dry nitrogen over heated magnesium at 800 °c: Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. Magnesium nitride. Magnesium Nitride + Water Equation.
From www.youtube.com
Equation for Mg(NO3)2 + H2O (Magnesium nitrate + Water) YouTube Magnesium Nitride + Water Equation By passing dry nitrogen over heated magnesium at 800 °c: Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. One mole of magnesium nitride [mg 3 n 2] and six moles of. Magnesium nitride + water = ammonia. Magnesium Nitride + Water Equation.
From h-o-m-e.org
What Is The Magnesium Nitride Formula? Magnesium Nitride + Water Equation Magnesium nitride + water = ammonia + magnesium oxide. Magnesium nitride + water = magnesium hydroxide + ammonia. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. 3 mg + n2 → mg3n2. 3 mg + 2 nh3 → mg3n2 + 3. For example, we have mg_3n_2 on the left side. Magnesium Nitride + Water Equation.
From www.youtube.com
Equation for Magnesium Hydroxide Dissolving in Water Mg(OH)2 + H2O Magnesium Nitride + Water Equation Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. 3 mg + n2 → mg3n2. By passing dry nitrogen over heated magnesium at 800 °c: Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED Write the chemical equation for the reaction of magnesium with Magnesium Nitride + Water Equation Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. 3 mg + 2 nh3 → mg3n2 + 3. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Magnesium nitride + water = ammonia + magnesium oxide.. Magnesium Nitride + Water Equation.
From wizedu.com
Solid magnesium nitride reacts with water to produce solid magnesium Magnesium Nitride + Water Equation Or ammonia at 700 °c: Magnesium nitride + water = magnesium hydroxide + ammonia. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. 3 mg + n2 → mg3n2. By passing dry nitrogen over heated magnesium at 800 °c: Mg3n2 + h2o = mg(oh)2 + nh3. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVEDQUESTION 12 Given the reaction 3Mg MgzN2 Whatis the unit Path Magnesium Nitride + Water Equation One mole of magnesium nitride [mg 3 n 2] and six moles of. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Magnesium nitride + water = magnesium hydroxide + ammonia. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. By passing dry nitrogen over heated magnesium at 800 °c: Or ammonia at 700. Magnesium Nitride + Water Equation.
From www.shutterstock.com
Magnesium Nitride Formula Handwritten Chemical Formula Stock Magnesium Nitride + Water Equation Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Or ammonia at 700 °c: 3 mg + 2 nh3 → mg3n2 + 3. One mole of magnesium nitride [mg 3 n 2] and six moles of. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Mg3n2 +. Magnesium Nitride + Water Equation.
From chrisnewslawrence.blogspot.com
What Is the Formula Mass of Magnesium Nitride Magnesium Nitride + Water Equation Magnesium nitride + water = ammonia + magnesium oxide. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. 3 mg + 2 nh3 → mg3n2 + 3. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Or ammonia at 700 °c: Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. 3 mg. Magnesium Nitride + Water Equation.
From www.studyxapp.com
the equation below shows the reaction between magnesium nitride and Magnesium Nitride + Water Equation Magnesium nitride + water = magnesium hydroxide + ammonia. By passing dry nitrogen over heated magnesium at 800 °c: For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. Mg3n2 + h2o = mg(oh)2 + nh3. Magnesium Nitride + Water Equation.
From chemicaldb.netlify.app
Molecular formula of magnesium nitrate Magnesium Nitride + Water Equation For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. 3 mg + 2 nh3 → mg3n2 + 3. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. 3 mg + n2 → mg3n2. By passing dry nitrogen over heated magnesium at 800 °c: In. Magnesium Nitride + Water Equation.
From www.nagwa.com
Question Video Balancing a Chemical Equation for the Reaction between Magnesium Nitride + Water Equation Magnesium nitride + water = magnesium hydroxide + ammonia. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. Magnesium nitride + water = ammonia + magnesium oxide. One mole of magnesium nitride [mg 3 n 2] and six moles of. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Mg3n2 + h2o = mg(oh)2. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED Magnesium metal can be combined directly with nitrogen to form Magnesium Nitride + Water Equation In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. By passing dry nitrogen over heated magnesium at 800 °c: Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. Mg3n2 + h2o = nh3 + mgo is a double. Magnesium Nitride + Water Equation.
From www.chegg.com
Solved Lithium nitride reacts with water to produce ammonia Magnesium Nitride + Water Equation By passing dry nitrogen over heated magnesium at 800 °c: Magnesium nitride + water = magnesium hydroxide + ammonia. 3 mg + n2 → mg3n2. One mole of magnesium nitride [mg 3 n 2] and six moles of. Magnesium nitride + water = ammonia + magnesium oxide. For example, we have mg_3n_2 on the left side and on the right. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVEDWhen magnesium metal is burned in air (Figure 3.6 ), two Magnesium Nitride + Water Equation Or ammonia at 700 °c: Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. 3 mg + n2 → mg3n2. Magnesium nitride + water = magnesium hydroxide + ammonia. 3 mg + 2 nh3 → mg3n2 + 3. One mole of magnesium nitride [mg 3. Magnesium Nitride + Water Equation.
From www.bartleby.com
Answered Lithium nitride reacts with water to… bartleby Magnesium Nitride + Water Equation By passing dry nitrogen over heated magnesium at 800 °c: For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. Or ammonia at 700 °c: Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. 3 mg + n2 → mg3n2. Magnesium nitride + water =. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED For the following reaction, 12.1 grams of magnesium nitride are Magnesium Nitride + Water Equation 3 mg + n2 → mg3n2. Magnesium nitride + water = ammonia + magnesium oxide. 3 mg + 2 nh3 → mg3n2 + 3. Or ammonia at 700 °c: In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. One mole. Magnesium Nitride + Water Equation.
From www.chegg.com
Solved Question 16 2 pts Magnesium hydroxide and ammonia Magnesium Nitride + Water Equation Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. Or ammonia at 700 °c: Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. 3 mg + 2 nh3 → mg3n2 + 3. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Mg3n2 + h2o = nh3 +. Magnesium Nitride + Water Equation.
From www.youtube.com
Mg(OH)2+HNO3=Mg(NO3)2+H2O Balanced EquationMagnesium Hydroxide+Nitric Magnesium Nitride + Water Equation In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Or ammonia at 700 °c: 3 mg + 2 nh3 → mg3n2 + 3. By passing dry nitrogen over heated magnesium at 800 °c: Magnesium nitride + water = magnesium hydroxide + ammonia. Mg3n2 + h2o = nh3 + mgo is a. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED 'Experiment 8 QUANTITIES IN CHEMICAL REACTIONS PreLab Magnesium Nitride + Water Equation Magnesium nitride + water = magnesium hydroxide + ammonia. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Magnesium nitride + water = ammonia + magnesium oxide. One mole of magnesium nitride [mg 3 n 2] and six moles of. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement.. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED Ammonia can be made by the reaction of water with magnesium Magnesium Nitride + Water Equation Or ammonia at 700 °c: In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Magnesium nitride + water = magnesium hydroxide + ammonia. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. Mg3n2 + h2o = nh3. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED For the following reaction, 0.579 moles of magnesium nitride Magnesium Nitride + Water Equation For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. By passing dry nitrogen over heated magnesium at 800 °c: In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Or ammonia at 700 °c: Mg3n2 + h2o =. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED Write the balanced equation for Magnesium nitride + water Magnesium Nitride + Water Equation By passing dry nitrogen over heated magnesium at 800 °c: 3 mg + n2 → mg3n2. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. One mole of magnesium nitride [mg 3 n 2] and. Magnesium Nitride + Water Equation.
From www.showme.com
Magnesium nitride empirical formula Science ShowMe Magnesium Nitride + Water Equation One mole of magnesium nitride [mg 3 n 2] and six moles of. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement.. Magnesium Nitride + Water Equation.
From ar.inspiredpencil.com
Magnesium And Water Magnesium Nitride + Water Equation Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. One mole of magnesium nitride [mg 3 n 2] and six moles of. Magnesium nitride + water =. Magnesium Nitride + Water Equation.
From www.chegg.com
Solved Solid magnesium nitride is placed in water and Magnesium Nitride + Water Equation Or ammonia at 700 °c: One mole of magnesium nitride [mg 3 n 2] and six moles of. 3 mg + 2 nh3 → mg3n2 + 3. In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. For example, we have mg_3n_2 on the left side and on the right side on. Magnesium Nitride + Water Equation.
From www.chegg.com
Solved Ammonia can be made by reaction of water with Magnesium Nitride + Water Equation By passing dry nitrogen over heated magnesium at 800 °c: 3 mg + 2 nh3 → mg3n2 + 3. Magnesium nitride + water = ammonia + magnesium + hydrogen peroxide. For example, we have mg_3n_2 on the left side and on the right side on the original equation we see magnesium as mg(oh)_2. One mole of magnesium nitride [mg 3. Magnesium Nitride + Water Equation.
From www.numerade.com
SOLVED When magnesium metal is burned in air, most of it reacts with Magnesium Nitride + Water Equation In this video we'll balance the equation mg3n2 + h2o = mgo + nh3 and provide the correct. Mg3n2 + h2o = mg(oh)2 + nh3 is a double displacement. 3 mg + 2 nh3 → mg3n2 + 3. Mg3n2 + h2o = nh3 + mgo is a double displacement (metathesis) reaction. 3 mg + n2 → mg3n2. One mole of. Magnesium Nitride + Water Equation.