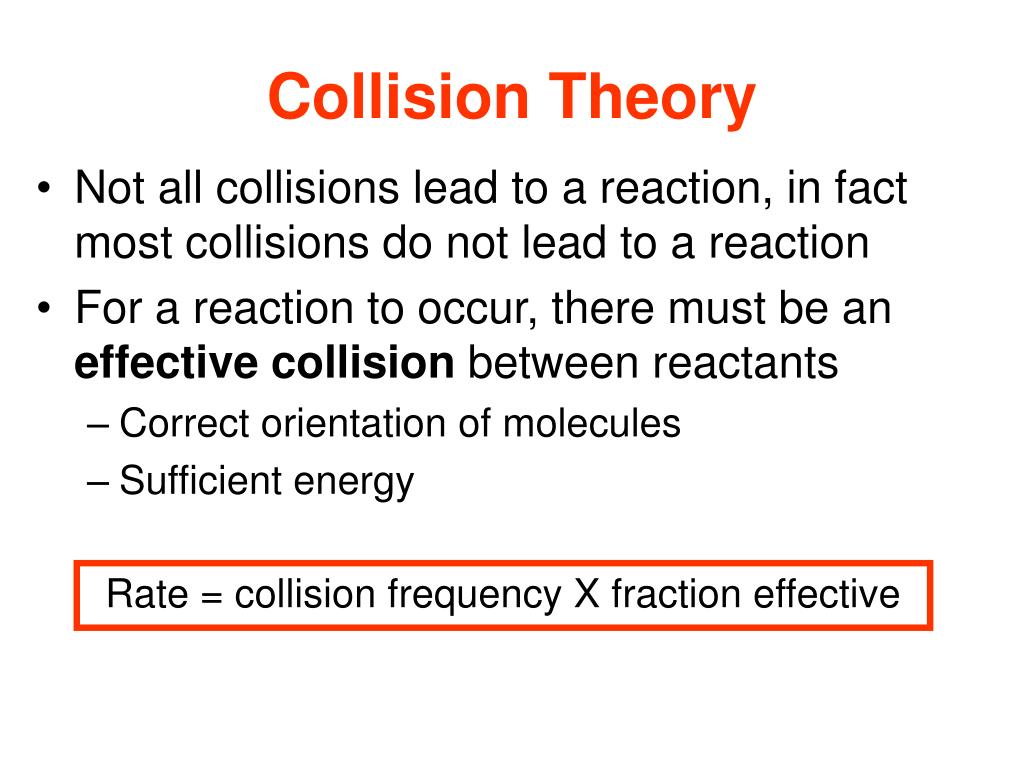
Collision Theory Affect Rate Of Reaction . Rates of reaction collision theory. The arrhenius equation describes the relation between a reaction’s. There are several factors that can affect the rate of a reaction. The rate of reaction measures how much product is made in a given. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Define activation energy and how it relates to energy diagrams. Describe collision theory and its connection to reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of the reaction depends. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Explaining rates using collision theory. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Chemical reactions vary in speed.
from www.slideserve.com
Explaining rates using collision theory. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of the reaction depends. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The arrhenius equation describes the relation between a reaction’s. The rate of reaction measures how much product is made in a given. Define activation energy and how it relates to energy diagrams. Describe collision theory and its connection to reaction rates. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. There are several factors that can affect the rate of a reaction.
PPT Collision Theory & Factors Affecting Reaction Rate PowerPoint
Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Describe collision theory and its connection to reaction rates. There are several factors that can affect the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of the reaction depends. Chemical reactions vary in speed. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The arrhenius equation describes the relation between a reaction’s. Rates of reaction collision theory. The rate of reaction measures how much product is made in a given. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Explaining rates using collision theory. Define activation energy and how it relates to energy diagrams.
From www.youtube.com
Collision theory in the rate of reaction YouTube Collision Theory Affect Rate Of Reaction Rates of reaction collision theory. There are several factors that can affect the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The rate of the reaction depends.. Collision Theory Affect Rate Of Reaction.
From writeatopic.com
How does the collision theory affect the rate of reaction? Write A Topic Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of the reaction. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT Ch. 18—Reaction Rates and Equilibrium PowerPoint Presentation Collision Theory Affect Rate Of Reaction Rates of reaction collision theory. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Explaining rates using collision theory. There are several factors that can affect the rate of a reaction. The rate of the reaction depends. Chemical reactions vary in speed. Describe collision theory and its connection to reaction rates.. Collision Theory Affect Rate Of Reaction.
From www.youtube.com
Lesson 6.2 Collision Theory and Factors Affecting Rates of Reaction Collision Theory Affect Rate Of Reaction The rate of the reaction depends. Rates of reaction collision theory. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Describe collision theory and its connection to reaction rates. The arrhenius equation describes the relation between a reaction’s. Define activation energy and how it relates to energy diagrams. There are several. Collision Theory Affect Rate Of Reaction.
From www.scribd.com
Factors That Affect Chemical Reaction Rates An Analysis of Collision Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of the reaction depends. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Rates of reaction collision theory. Use the postulates of collision theory to explain the effects of physical state,. Collision Theory Affect Rate Of Reaction.
From www.nagwa.com
Question Video Recalling How Parameters in Collision Theory Affect Collision Theory Affect Rate Of Reaction Explaining rates using collision theory. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Rates of reaction collision theory. The arrhenius equation describes the relation between a reaction’s. The rate of the reaction depends. Chemical reactions vary in speed. Collision theory provides a simple but effective explanation for the effect of. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT Collision Theory PowerPoint Presentation, free download ID3873529 Collision Theory Affect Rate Of Reaction Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The arrhenius equation describes the relation between a reaction’s. Chemical reactions vary in speed. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Explaining rates using collision theory. Collision theory states that. Collision Theory Affect Rate Of Reaction.
From slideplayer.com
Collision Theory Factors affecting rate Particle size affects rate Collision Theory Affect Rate Of Reaction There are several factors that can affect the rate of a reaction. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect. Collision Theory Affect Rate Of Reaction.
From slideplayer.com
Reaction Rates Reaction Rates Collision Theory ppt download Collision Theory Affect Rate Of Reaction Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The rate of the reaction depends. Describe collision theory and its connection to reaction rates. Chemical reactions vary in speed. The arrhenius equation describes the relation between a reaction’s. Rates of reaction collision theory. Explaining rates using collision theory. Collision theory. Collision Theory Affect Rate Of Reaction.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Theory Affect Rate Of Reaction There are several factors that can affect the rate of a reaction. Explaining rates using collision theory. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Rates of reaction collision theory. Define activation energy and how it relates to energy diagrams. The rate of reaction measures how much product is made. Collision Theory Affect Rate Of Reaction.
From www.youtube.com
Rate02Surface area affects rate, collision theory IGCSE/GCSE Collision Theory Affect Rate Of Reaction Rates of reaction collision theory. There are several factors that can affect the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Describe collision theory and its connection to reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Collision Theory Affect Rate Of Reaction Define activation energy and how it relates to energy diagrams. The rate of the reaction depends. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The arrhenius equation describes the relation between a reaction’s. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on. Collision Theory Affect Rate Of Reaction.
From www.vecteezy.com
collision theory of reaction rate 27798497 Vector Art at Vecteezy Collision Theory Affect Rate Of Reaction The rate of reaction measures how much product is made in a given. Explaining rates using collision theory. The rate of the reaction depends. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. There are several factors that can affect the rate of a reaction. Collision theory provides a simple but. Collision Theory Affect Rate Of Reaction.
From www.chemistrystudent.com
Collision Theory (ALevel) ChemistryStudent Collision Theory Affect Rate Of Reaction Chemical reactions vary in speed. The rate of reaction measures how much product is made in a given. Rates of reaction collision theory. The rate of the reaction depends. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The arrhenius equation describes the relation between a reaction’s. Describe collision theory and. Collision Theory Affect Rate Of Reaction.
From dokumen.tips
(PPTX) Ch18.1 Reaction Rates Reaction Rates explained by collision Collision Theory Affect Rate Of Reaction There are several factors that can affect the rate of a reaction. The rate of reaction measures how much product is made in a given. The rate of the reaction depends. Define activation energy and how it relates to energy diagrams. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. Collision Theory Affect Rate Of Reaction.
From www.youtube.com
Reaction Rate & Collision Theory // Preliminary HSC Chemistry YouTube Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define activation energy and how it relates to energy diagrams. Explaining rates using collision theory. Rates of reaction collision theory. The rate of reaction. Collision Theory Affect Rate Of Reaction.
From mmerevise.co.uk
Collision Theory and Reaction Rates MME Collision Theory Affect Rate Of Reaction There are several factors that can affect the rate of a reaction. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. The rate of the reaction depends. Explaining rates using collision theory. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. Collision Theory Affect Rate Of Reaction.
From slideplayer.com
Chapter 1 Rate of Reaction. ppt download Collision Theory Affect Rate Of Reaction Define activation energy and how it relates to energy diagrams. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Chemical reactions vary in speed. Describe collision theory and its connection to reaction rates. The arrhenius equation describes the relation between a reaction’s. Explaining rates using collision theory. Collision theory provides. Collision Theory Affect Rate Of Reaction.
From saylordotorg.github.io
The Collision Model of Chemical Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Explaining rates using collision theory. Describe collision theory and its connection to reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Collision theory states that for a chemical reaction to. Collision Theory Affect Rate Of Reaction.
From integratedsciencegeneral11.weebly.com
Collision Theory Explains Chemical Reactions Integrated Science 11 Collision Theory Affect Rate Of Reaction Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Explaining rates using collision theory. Describe collision theory and its connection to reaction rates. Rates of reaction collision theory. Chemical reactions vary in. Collision Theory Affect Rate Of Reaction.
From mrtremblaycambridge.weebly.com
C7. Chemical reactions Mr. Tremblay's Class Site Collision Theory Affect Rate Of Reaction There are several factors that can affect the rate of a reaction. Define activation energy and how it relates to energy diagrams. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The rate of reaction measures how much product is made in a given. Collision theory states that for a. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT RATES OF REACTION A guide for GCSE students PowerPoint Collision Theory Affect Rate Of Reaction Define activation energy and how it relates to energy diagrams. Describe collision theory and its connection to reaction rates. Explaining rates using collision theory. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction. Collision Theory Affect Rate Of Reaction.
From blogs.glowscotland.org.uk
Collision Theory and Rate of Reaction Higher Chemistry Unit 1 Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Rates of reaction collision theory. The arrhenius equation describes the relation between a reaction’s. There are several factors that can affect the rate of. Collision Theory Affect Rate Of Reaction.
From www.youtube.com
Factors That Affect Reaction Rate Collision Theory, Activation Energy Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Explaining rates using collision theory. There are several factors that can affect the rate of a reaction. Chemical reactions vary in speed. The rate. Collision Theory Affect Rate Of Reaction.
From www.slideshare.net
Rate of reaction(f5) Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of reaction measures how much product is made in a given. The arrhenius equation describes the relation between a reaction’s. Chemical reactions vary in speed. The rate of the reaction depends. Define activation energy and how it relates to energy. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT Reaction Rates and Equilibrium PowerPoint Presentation, free Collision Theory Affect Rate Of Reaction There are several factors that can affect the rate of a reaction. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The arrhenius equation describes the relation between a reaction’s. Rates of. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT Collision Theory and Reaction Rates PowerPoint Presentation, free Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Explaining rates using collision theory. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT Collision Theory and Reaction Rate PowerPoint Presentation, free Collision Theory Affect Rate Of Reaction Chemical reactions vary in speed. The rate of the reaction depends. Explaining rates using collision theory. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Describe collision theory and its connection to reaction rates. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with. Collision Theory Affect Rate Of Reaction.
From mmerevise.co.uk
Factors Affecting the Rate of Reaction Revision MME Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The arrhenius equation describes the relation between a reaction’s. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The rate of the reaction depends. Rates of reaction collision theory. Define activation energy. Collision Theory Affect Rate Of Reaction.
From www.youtube.com
Factors Affecting Rate of Reaction + Collision Theory GCSE Science Collision Theory Affect Rate Of Reaction Explaining rates using collision theory. Collision theory states that for a chemical reaction to occur, the reacting particles must collide with one another. Define activation energy and how it relates to energy diagrams. Chemical reactions vary in speed. The arrhenius equation describes the relation between a reaction’s. The rate of reaction measures how much product is made in a given.. Collision Theory Affect Rate Of Reaction.
From materiallibethel.z13.web.core.windows.net
Collision Theory And Rate Of Reaction Collision Theory Affect Rate Of Reaction There are several factors that can affect the rate of a reaction. The rate of reaction measures how much product is made in a given. Rates of reaction collision theory. Describe collision theory and its connection to reaction rates. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Define activation energy. Collision Theory Affect Rate Of Reaction.
From www.slideserve.com
PPT Collision Theory & Factors Affecting Reaction Rate PowerPoint Collision Theory Affect Rate Of Reaction Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates Define activation energy and how it relates to energy diagrams. Rates of reaction collision theory. The rate of the reaction depends. The rate of reaction measures how much product is made in a given. The arrhenius equation describes the relation between. Collision Theory Affect Rate Of Reaction.
From pablogroeaton.blogspot.com
Collision Theory of Reaction Rates PablogroEaton Collision Theory Affect Rate Of Reaction The rate of reaction measures how much product is made in a given. Explaining rates using collision theory. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Describe collision theory and its connection to reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and. Collision Theory Affect Rate Of Reaction.
From materiallibstaudt.z13.web.core.windows.net
Collision Theory Of Reactions Collision Theory Affect Rate Of Reaction Describe collision theory and its connection to reaction rates. Use the postulates of collision theory to explain the effects of physical state, temperature, and concentration on reaction rates The rate of the reaction depends. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Collision theory provides a simple but effective explanation. Collision Theory Affect Rate Of Reaction.
From www.youtube.com
Collision Theory / Reaction Rate YouTube Collision Theory Affect Rate Of Reaction Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. The rate of reaction measures how much product is made in a given. Collision theory provides a simple but effective explanation for the effect of many experimental parameters on reaction rates. Use the postulates of collision theory to explain the effects of. Collision Theory Affect Rate Of Reaction.