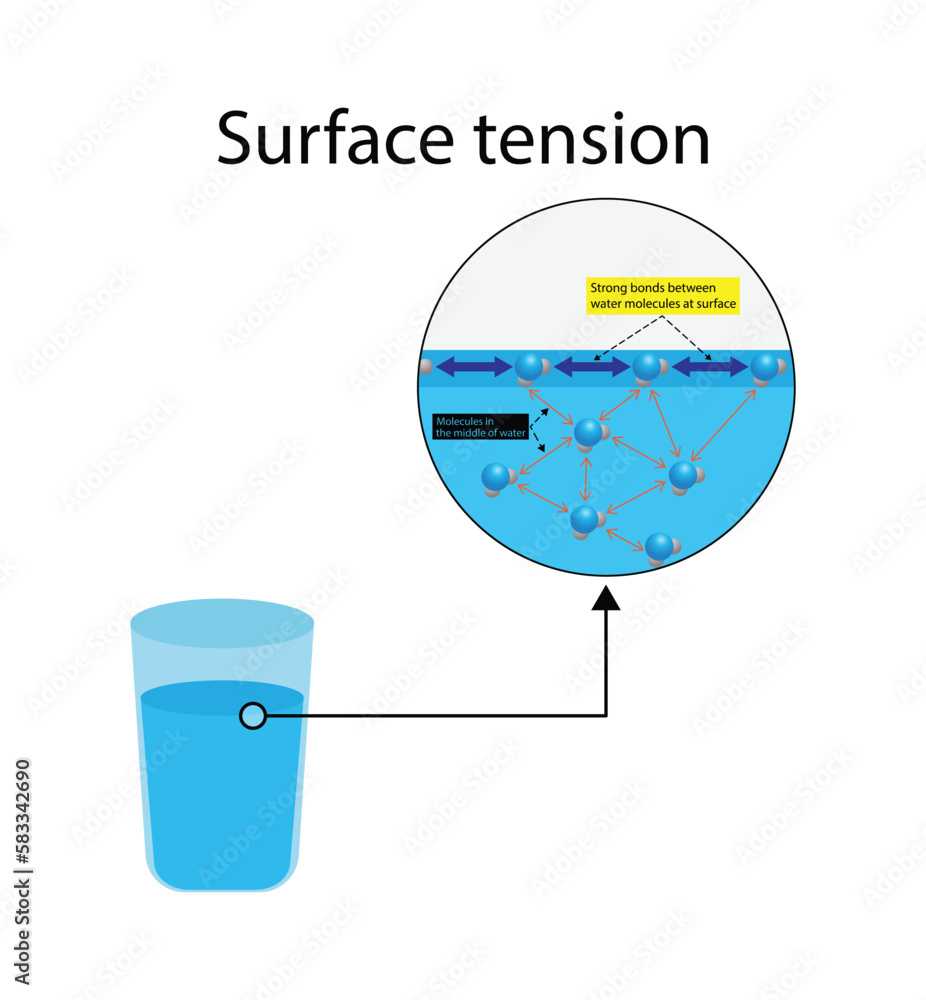
What Does Water Have A Greater Surface Tension Than Alcohol . • the attraction of molecules at the surface of a liquid is called surface tension. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Water, soapy water, or rubbing alcohol. • the polarity of water molecules can help explain why water. In comparison, organic liquids, such as benzene and alcohols,. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The liquid that can fit the most droplets onto the. It will then take a certain amount. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid.
from stock.adobe.com
The liquid that can fit the most droplets onto the. In comparison, organic liquids, such as benzene and alcohols,. • the polarity of water molecules can help explain why water. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. It will then take a certain amount. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. Water, soapy water, or rubbing alcohol. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface.
illustration of physics, Surface tension of water, the cohesive forces
What Does Water Have A Greater Surface Tension Than Alcohol To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Water, soapy water, or rubbing alcohol. In comparison, organic liquids, such as benzene and alcohols,. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. It will then take a certain amount. • the attraction of molecules at the surface of a liquid is called surface tension. The liquid that can fit the most droplets onto the. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. • the polarity of water molecules can help explain why water. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Does Water Have A Greater Surface Tension Than Alcohol • the polarity of water molecules can help explain why water. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water, soapy water, or rubbing alcohol. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. • the attraction of molecules at the surface of. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.expii.com
Surface Tension of Water — Overview & Importance Expii What Does Water Have A Greater Surface Tension Than Alcohol To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. • the polarity of water molecules can help explain why water. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The interaction between a molecule of water and an ion is. What Does Water Have A Greater Surface Tension Than Alcohol.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces What Does Water Have A Greater Surface Tension Than Alcohol Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The liquid that can fit the most droplets onto the. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Water has a surface tension of 0.07275 joule per. What Does Water Have A Greater Surface Tension Than Alcohol.
From animalia-life.club
Water Molecule Structure For Kids What Does Water Have A Greater Surface Tension Than Alcohol Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.acs.org
Simulations & Videos for Lesson 5.2 Surface Tension American What Does Water Have A Greater Surface Tension Than Alcohol Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). • the polarity of water molecules can help explain why water. • the attraction of molecules at the surface of a liquid is called surface tension. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.pinterest.com
Cartoon depiction of water as a solid, liquid, and a gas. Wax paper What Does Water Have A Greater Surface Tension Than Alcohol Water, soapy water, or rubbing alcohol. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). It will then take a certain amount. The interaction between a molecule of water and an. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.youtube.com
Surface tension of liquid water vs. Alcohol YouTube What Does Water Have A Greater Surface Tension Than Alcohol It will then take a certain amount. • the polarity of water molecules can help explain why water. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.slideserve.com
PPT Properties of Liquids PowerPoint Presentation, free download ID What Does Water Have A Greater Surface Tension Than Alcohol The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. Water, soapy. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Does Water Have A Greater Surface Tension Than Alcohol Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. In comparison, organic liquids, such as benzene and alcohols,. There are forces between the liquid such as van der waals. What Does Water Have A Greater Surface Tension Than Alcohol.
From studyfullirene.z21.web.core.windows.net
Surface Tension Of Water Worksheet What Does Water Have A Greater Surface Tension Than Alcohol Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). It will then take a certain amount. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. There are forces between the liquid such as van der waals forces that are responsible. What Does Water Have A Greater Surface Tension Than Alcohol.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Does Water Have A Greater Surface Tension Than Alcohol The liquid that can fit the most droplets onto the. Water, soapy water, or rubbing alcohol. • the attraction of molecules at the surface of a liquid is called surface tension. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. There are forces between the liquid such as van der waals. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Does Water Have A Greater Surface Tension Than Alcohol The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. • the attraction of molecules at the surface of a liquid is called surface tension.. What Does Water Have A Greater Surface Tension Than Alcohol.
From celxworo.blob.core.windows.net
Surface Tension Of Water Vs Alcohol at Amy Conner blog What Does Water Have A Greater Surface Tension Than Alcohol Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. The liquid that can fit the most droplets onto the. Water, soapy water, or rubbing alcohol. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). To do this, you will count the number of droplets. What Does Water Have A Greater Surface Tension Than Alcohol.
From slideplayer.com
Water Chapter ppt download What Does Water Have A Greater Surface Tension Than Alcohol To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. • the polarity of water molecules can help explain why water. In comparison, organic liquids, such. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.slideserve.com
PPT LIQUID SURFACES PowerPoint Presentation, free download ID4456243 What Does Water Have A Greater Surface Tension Than Alcohol In comparison, organic liquids, such as benzene and alcohols,. It will then take a certain amount. Water, soapy water, or rubbing alcohol. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.youtube.com
Surface Tension Water Alcohol YouTube What Does Water Have A Greater Surface Tension Than Alcohol To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. It will then take a certain amount. In comparison, organic liquids, such as benzene and alcohols,. Water, soapy water, or. What Does Water Have A Greater Surface Tension Than Alcohol.
From slidetodoc.com
WATER WATER THE MOST ABUNDANT COMPOUND ON EARTH What Does Water Have A Greater Surface Tension Than Alcohol Water, soapy water, or rubbing alcohol. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. To do this, you will count the number of droplets that can fit onto a penn without spilling over for. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.slideserve.com
PPT Physical Pharmacy SURFACE TENSION PowerPoint Presentation, free What Does Water Have A Greater Surface Tension Than Alcohol For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. • the attraction of molecules at the surface of a liquid is called surface tension. In comparison, organic liquids, such as benzene and alcohols,. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.researchgate.net
Surface tension of ethanolwater binary mixtures as a function of f e What Does Water Have A Greater Surface Tension Than Alcohol The liquid that can fit the most droplets onto the. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. • the attraction of molecules at the surface of a liquid is called surface tension. Water has a surface tension of 0.07275 joule per square metre at 20. What Does Water Have A Greater Surface Tension Than Alcohol.
From slideplayer.com
Water Chapter ppt download What Does Water Have A Greater Surface Tension Than Alcohol It will then take a certain amount. • the attraction of molecules at the surface of a liquid is called surface tension. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. • the polarity of water molecules can help explain why water. To do this, you will count the. What Does Water Have A Greater Surface Tension Than Alcohol.
From celxworo.blob.core.windows.net
Surface Tension Of Water Vs Alcohol at Amy Conner blog What Does Water Have A Greater Surface Tension Than Alcohol For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. Water, soapy water, or rubbing alcohol. In comparison, organic liquids, such as benzene and alcohols,. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. • the polarity. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.slideserve.com
PPT Water and Aqueous Systems PowerPoint Presentation, free download What Does Water Have A Greater Surface Tension Than Alcohol The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. For example, water, with its strong intermolecular hydrogen bonding, has one of the highest surface tension values of any liquid,. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.slideserve.com
PPT Chapter 11 Intermolecular Forces, Liquids, and Solids PowerPoint What Does Water Have A Greater Surface Tension Than Alcohol The liquid that can fit the most droplets onto the. In comparison, organic liquids, such as benzene and alcohols,. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. It will then take a certain amount. • the polarity of water molecules can help explain why water. Water, soapy water, or rubbing. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.slideserve.com
PPT Chapter 15 PowerPoint Presentation, free download ID245553 What Does Water Have A Greater Surface Tension Than Alcohol It will then take a certain amount. To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Water has a surface tension of 0.07275 joule. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.learnatnoon.com
The boiling point of water and alcohol explained Noon Academy What Does Water Have A Greater Surface Tension Than Alcohol Water, soapy water, or rubbing alcohol. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. The liquid that can fit the most droplets onto the. In comparison, organic liquids, such as benzene and alcohols,. Water has a high surface tension because hydrogen bonds among water molecules resist. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.researchgate.net
Change of surface tension of aqueous ethanol solution as a function of What Does Water Have A Greater Surface Tension Than Alcohol There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The liquid that can fit the most droplets onto the. Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. For example, water, with its strong intermolecular hydrogen bonding,. What Does Water Have A Greater Surface Tension Than Alcohol.
From exobarygp.blob.core.windows.net
Measurement Of Surface Tension And Viscosity at Vicky Quin blog What Does Water Have A Greater Surface Tension Than Alcohol Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). The liquid that can fit the most droplets onto the. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. Water, soapy water, or rubbing alcohol. Water has a high surface tension. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.slideserve.com
PPT Chapter 3 Water and Life PowerPoint Presentation, free download What Does Water Have A Greater Surface Tension Than Alcohol • the attraction of molecules at the surface of a liquid is called surface tension. It will then take a certain amount. In comparison, organic liquids, such as benzene and alcohols,. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. Water has a high surface tension because. What Does Water Have A Greater Surface Tension Than Alcohol.
From ideal.accelerate-ed.com
Surface Tension What Does Water Have A Greater Surface Tension Than Alcohol • the polarity of water molecules can help explain why water. • the attraction of molecules at the surface of a liquid is called surface tension. It will then take a certain amount. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. Water has a high. What Does Water Have A Greater Surface Tension Than Alcohol.
From celxworo.blob.core.windows.net
Surface Tension Of Water Vs Alcohol at Amy Conner blog What Does Water Have A Greater Surface Tension Than Alcohol Water, soapy water, or rubbing alcohol. It will then take a certain amount. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. • the. What Does Water Have A Greater Surface Tension Than Alcohol.
From www.youtube.com
Surface Tension of Water Explained YouTube What Does Water Have A Greater Surface Tension Than Alcohol To do this, you will count the number of droplets that can fit onto a penn without spilling over for each liquid. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The interaction between a molecule of water and an ion is stronger than the hydrogen. What Does Water Have A Greater Surface Tension Than Alcohol.
From slideplayer.com
Chapter 10 States of Matter & Water Cycle ppt download What Does Water Have A Greater Surface Tension Than Alcohol Water has a high surface tension because hydrogen bonds among water molecules resist stretching or breaking the surface. Water has a surface tension of 0.07275 joule per square metre at 20 °c (68 °f). In comparison, organic liquids, such as benzene and alcohols,. There are forces between the liquid such as van der waals forces that are responsible for the. What Does Water Have A Greater Surface Tension Than Alcohol.
From exolmohec.blob.core.windows.net
How Does Water Have High Surface Tension at Shelton Nicholson blog What Does Water Have A Greater Surface Tension Than Alcohol There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. In comparison, organic liquids, such as benzene and alcohols,. • the polarity of water molecules. What Does Water Have A Greater Surface Tension Than Alcohol.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Does Water Have A Greater Surface Tension Than Alcohol • the attraction of molecules at the surface of a liquid is called surface tension. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. There are forces between the liquid such as van der waals forces that are responsible for the intermolecular forces found within the liquid.. What Does Water Have A Greater Surface Tension Than Alcohol.
From 88guru.com
Viscosity And Surface Tension Definition, Applications and FAQ's 88Guru What Does Water Have A Greater Surface Tension Than Alcohol • the polarity of water molecules can help explain why water. It will then take a certain amount. Water, soapy water, or rubbing alcohol. The interaction between a molecule of water and an ion is stronger than the hydrogen bonding that occurs between two water molecules. Water has a surface tension of 0.07275 joule per square metre at 20 °c. What Does Water Have A Greater Surface Tension Than Alcohol.