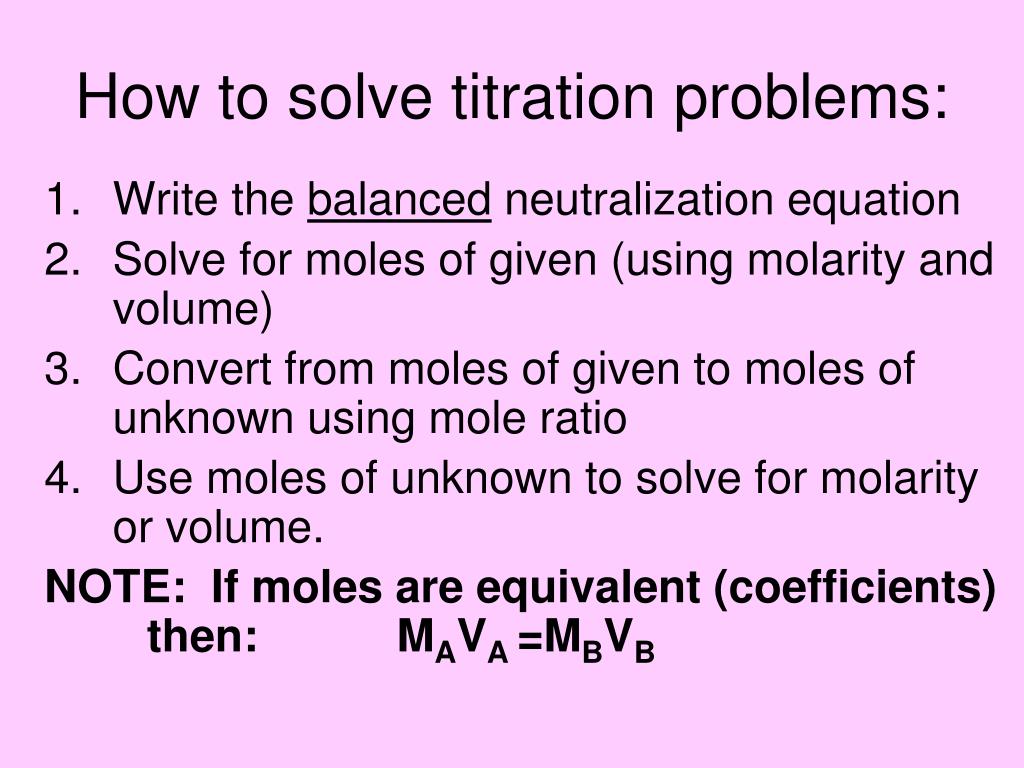
Titration Lab Problems . A titration is an analytical procedure used to determine the accurate concentration of a sample by. Learn how to prepare a standard solution, calculate. Be able to determine the k a or k b from ph data associated with the titration. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. You will also learn about equivalence points. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. what is a titration?
from www.slideserve.com
Be able to determine the k a or k b from ph data associated with the titration. what is a titration? Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Learn how to prepare a standard solution, calculate. A titration is an analytical procedure used to determine the accurate concentration of a sample by. You will also learn about equivalence points. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
PPT Titrations PowerPoint Presentation, free download ID2145706
Titration Lab Problems You will also learn about equivalence points. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. Be able to determine the k a or k b from ph data associated with the titration. You will also learn about equivalence points. Learn how to prepare a standard solution, calculate. what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one.
From www.youtube.com
TITRATION SAMPLE PROBLEMS YouTube Titration Lab Problems in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. what is a titration? in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Learn how to prepare a standard solution, calculate.. Titration Lab Problems.
From learningschoolregibanb.z21.web.core.windows.net
Chemistry Titration Questions And Answers Titration Lab Problems Be able to determine the k a or k b from ph data associated with the titration. Learn how to prepare a standard solution, calculate. A titration is an analytical procedure used to determine the accurate concentration of a sample by. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and. Titration Lab Problems.
From www.showme.com
Titration calculations Science, Chemistry, Chemicalreactions Titration Lab Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Be able to determine the k a or k b from ph data associated with. Titration Lab Problems.
From www.youtube.com
Stoichiometry Problem Titration Calculation YouTube Titration Lab Problems A titration is an analytical procedure used to determine the accurate concentration of a sample by. You will also learn about equivalence points. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Be able to determine the k a or k. Titration Lab Problems.
From www.youtube.com
Titration Lab AP Chem YouTube Titration Lab Problems You will also learn about equivalence points. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Learn how to prepare a standard solution, calculate. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is an analytical. Titration Lab Problems.
From www.pinterest.com
titration problems Teaching chemistry, Chemistry lessons, High school Titration Lab Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. what is a titration? You will also learn about equivalence points. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this. Titration Lab Problems.
From www.ck12.org
Titration (Calculations) Example 2 ( Video ) Chemistry CK12 Titration Lab Problems Learn how to prepare a standard solution, calculate. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. the above equation works only for neutralizations in. Titration Lab Problems.
From ebinu.blog
AcidBase Titration Lab — DataClassroom / Acid Base Titration Titration Lab Problems in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. A titration is an analytical procedure used to. Titration Lab Problems.
From dxovbottd.blob.core.windows.net
Titration Experiment Notes at Victor Shoemaker blog Titration Lab Problems Be able to determine the k a or k b from ph data associated with the titration. You will also learn about equivalence points. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Learn how to prepare a standard solution, calculate. what is a titration? in. Titration Lab Problems.
From ceiuntct.blob.core.windows.net
Hcl And Na2Co3 Titration Calculations at Dorothy Ducharme blog Titration Lab Problems Learn how to prepare a standard solution, calculate. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. Be able to determine the k a or k b from ph data associated with the titration. what is a titration? in this tutorial, you will. Titration Lab Problems.
From chp090.chemistry.wustl.edu
Redox Titration Problem Titration Lab Problems the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. what is a titration? Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m. Titration Lab Problems.
From mungfali.com
Titration Steps Titration Lab Problems what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. in this tutorial, you will learn about titration curves, titration analysis and the steps. Titration Lab Problems.
From studylib.net
Titration Practice Worksheet Titration Lab Problems the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. A titration is an analytical procedure used to determine the accurate concentration of a sample by. Be able to determine the k a or k b from ph data associated with the titration. in this section, we will. Titration Lab Problems.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration Lab Problems You will also learn about equivalence points. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. A. Titration Lab Problems.
From dxooyumci.blob.core.windows.net
Titration Practice Problems Chemistry at Gloria Ogorman blog Titration Lab Problems Learn how to prepare a standard solution, calculate. You will also learn about equivalence points. what is a titration? in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Be able to determine the k a or k b from ph data associated with the titration. the above equation. Titration Lab Problems.
From cewuuttv.blob.core.windows.net
Titration Math Problems at Joy Rodriguez blog Titration Lab Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. the above equation works only for neutralizations. Titration Lab Problems.
From www.youtube.com
Titration (sample problems) YouTube Titration Lab Problems A titration is an analytical procedure used to determine the accurate concentration of a sample by. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. You will also learn. Titration Lab Problems.
From philo-khosravani.blogspot.com
Acid Base Titration Worksheet Answers philokhosravani Titration Lab Problems what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. You will also learn about equivalence points. Be able to determine. Titration Lab Problems.
From www.slideserve.com
PPT Titrations PowerPoint Presentation, free download ID2145706 Titration Lab Problems A titration is an analytical procedure used to determine the accurate concentration of a sample by. You will also learn about equivalence points. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq). Titration Lab Problems.
From www.ck12.org
Titration (Calculations) Example 3 ( Video ) Chemistry CK12 Titration Lab Problems in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. what is a titration? Be able to determine the k a or k b from ph. Titration Lab Problems.
From studylib.net
acid base titration worksheet answer key Titration Lab Problems You will also learn about equivalence points. Learn how to prepare a standard solution, calculate. A titration is an analytical procedure used to determine the accurate concentration of a sample by. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. the above equation works only for neutralizations in which. Titration Lab Problems.
From www.youtube.com
Solving AcidBase Titration Problems YouTube Titration Lab Problems the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. what is a titration? Be able to determine the k a or k b from ph data associated with the titration. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3. Titration Lab Problems.
From www.scribd.com
titration ( chemistry Experiment report) Titration Analysis Titration Lab Problems in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. the above equation works only for neutralizations in which there is a 1:1 ratio between the. Titration Lab Problems.
From chem4three.blogspot.com
CHEMISTRY 11 TITRATIONS Titration Lab Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. You will also learn about equivalence points. Be able to determine the k a or k b from ph data associated with the titration. A titration is an analytical procedure used to determine the accurate concentration. Titration Lab Problems.
From exoaelzfm.blob.core.windows.net
Titration Problems Pdf at Clifton Gomez blog Titration Lab Problems Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. Be able to determine the k a or k b from ph data associated with the titration. You will also learn about equivalence points. A titration is an analytical procedure used to determine the accurate concentration. Titration Lab Problems.
From cecpajsp.blob.core.windows.net
How To Calculate Titration Problems at Gerald Bates blog Titration Lab Problems in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. what is a titration? A titration is an analytical procedure used to determine the accurate concentration of a sample by. Be able to determine the k a or k b from ph data associated with the titration. the above. Titration Lab Problems.
From www.youtube.com
Acid Base Titration Curves pH Calculations YouTube Titration Lab Problems You will also learn about equivalence points. the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. what is a titration? Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. in this. Titration Lab Problems.
From mavink.com
Acid Base Titration Lab Procedure Titration Lab Problems in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. A titration is an analytical procedure used to determine the accurate concentration of a sample by. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid. Titration Lab Problems.
From lessonlibmasters.z21.web.core.windows.net
Titration Practice Problems With Answers Pdf Titration Lab Problems Be able to determine the k a or k b from ph data associated with the titration. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. what is a titration? You will also learn about equivalence points. in this section, we will see. Titration Lab Problems.
From www.studocu.com
Experiment 7 Titrations Experiment 7 Titrations Required reading Titration Lab Problems Learn how to prepare a standard solution, calculate. what is a titration? the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or. Titration Lab Problems.
From www.youtube.com
AcidBase Titration Equivalence Point YouTube Titration Lab Problems You will also learn about equivalence points. what is a titration? Be able to determine the k a or k b from ph data associated with the titration. in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. A titration is. Titration Lab Problems.
From learningschoolmurgkerny1.z13.web.core.windows.net
Titration Questions And Answers Pdf A Level Titration Lab Problems in this section, we will see how to perform calculations to predict the ph at any point in a titration of a weak acid or base, using. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. the above equation works only for neutralizations. Titration Lab Problems.
From printablehaferbrotwp.z21.web.core.windows.net
How To Do Titrations In Chemistry Titration Lab Problems You will also learn about equivalence points. what is a titration? Learn how to prepare a standard solution, calculate. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. the above equation works only for neutralizations in which there is a 1:1 ratio between. Titration Lab Problems.
From exojqptkh.blob.core.windows.net
Titration Flask Titrant at Jodi Goldberg blog Titration Lab Problems the above equation works only for neutralizations in which there is a 1:1 ratio between the acid and the base. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno 3 (aq) and 0.200 m naoh (titrant) at. A titration is an analytical procedure used to determine the accurate concentration of a sample. Titration Lab Problems.
From www.showme.com
Titration problems Science, Chemistry ShowMe Titration Lab Problems Learn how to prepare a standard solution, calculate. Be able to determine the k a or k b from ph data associated with the titration. in this tutorial, you will learn about titration curves, titration analysis and the steps required to perform one. Calculate the ph for the strong acid/strong base titration between 50.0 ml of 0.100 m hno. Titration Lab Problems.