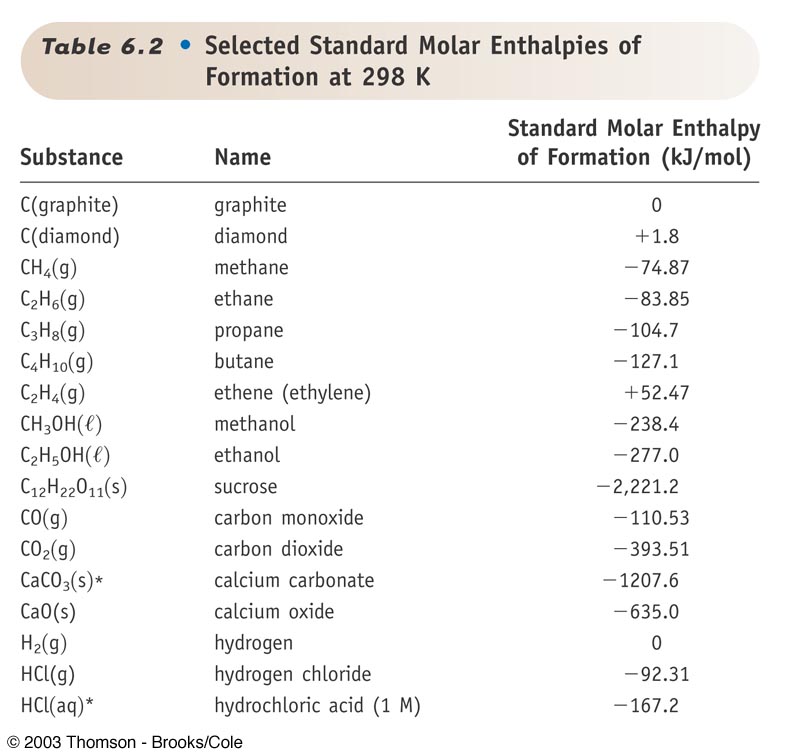
Standard Enthalpy Of Formation Of H2 . A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. All standard state, 25 °c and 1 bar (written to 1 decimal place). For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. When would one use it in a calculation of enthalpy change? Mallard, eds, nist chemistry webbook, nist standard. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. The standard enthalpy of formation of any element in its standard state is zero by definition. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
from lessonluft.z19.web.core.windows.net
Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Mallard, eds, nist chemistry webbook, nist standard.
Heat Of Formation Chart
Standard Enthalpy Of Formation Of H2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Mallard, eds, nist chemistry webbook, nist standard. The standard enthalpy of formation of any element in its standard state is zero by definition. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. All standard state, 25 °c and 1 bar (written to 1 decimal place). A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. When would one use it in a calculation of enthalpy change? Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.
From www.writework.com
A comparison between the Enthalpy of formation of MgO acquired via a Standard Enthalpy Of Formation Of H2 The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound. Standard Enthalpy Of Formation Of H2.
From www.chem.fsu.edu
CHM1045 Enthalpy Lecture Standard Enthalpy Of Formation Of H2 Mallard, eds, nist chemistry webbook, nist standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation of. Standard Enthalpy Of Formation Of H2.
From classnotes.org.in
Enthalpies Of Reaction Chemistry, Class 11, Thermodynamics Standard Enthalpy Of Formation Of H2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For. Standard Enthalpy Of Formation Of H2.
From mavink.com
Enthalpy Of Formation Equation Standard Enthalpy Of Formation Of H2 From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. All standard state, 25 °c and 1 bar (written to 1 decimal place). Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. When would one use it in a calculation of. Standard Enthalpy Of Formation Of H2.
From rayb78.github.io
Heat Of Formation Chart Standard Enthalpy Of Formation Of H2 A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. When would one use it in a calculation of enthalpy change? 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of.. Standard Enthalpy Of Formation Of H2.
From www.linstitute.net
Edexcel A Level Chemistry复习笔记1.8.1 Enthalpy Changes翰林国际教育 Standard Enthalpy Of Formation Of H2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A standard enthalpy of formation $δh°_f$ is an enthalpy. Standard Enthalpy Of Formation Of H2.
From schoolworkhelper.net
Standard Enthalpies of Formation, ΔH°f SchoolWorkHelper Standard Enthalpy Of Formation Of H2 When would one use it in a calculation of enthalpy change? For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. All standard state, 25 °c and 1 bar (written to 1. Standard Enthalpy Of Formation Of H2.
From www.chegg.com
Solved Standard Enthalpy Of Formation Of H2 The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. 193. Standard Enthalpy Of Formation Of H2.
From www.youtube.com
CHEM 101 Using Standard Enthalpies of Formation and Standard Enthalpy Standard Enthalpy Of Formation Of H2 From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. All standard state, 25 °c and 1 bar (written to 1 decimal place). When would one use. Standard Enthalpy Of Formation Of H2.
From askfilo.com
At 298 K the standard enthalpies of formation of H2 O(I) and H2 O2 (I) ar.. Standard Enthalpy Of Formation Of H2 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is defined. Standard Enthalpy Of Formation Of H2.
From jordanhumphries.z13.web.core.windows.net
Enthalpy Of Formation Chart Standard Enthalpy Of Formation Of H2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy. Standard Enthalpy Of Formation Of H2.
From exozagkku.blob.core.windows.net
Standard Enthalpy Of Formation Symbol at Diane Downs blog Standard Enthalpy Of Formation Of H2 The standard enthalpy of formation of any element in its standard state is zero by definition. All standard state, 25 °c and 1 bar (written to 1 decimal place). A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Mallard, eds, nist chemistry webbook,. Standard Enthalpy Of Formation Of H2.
From mavink.com
Standard Enthalpy Of Combustion Chart Standard Enthalpy Of Formation Of H2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. From what i understand hydrogen only exists as a monatomic gas at very high temperatures, the standard. The. Standard Enthalpy Of Formation Of H2.
From www.slideserve.com
PPT STANDARD MOLAR ENTHALPY OF FORMATION PowerPoint Presentation Standard Enthalpy Of Formation Of H2 The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Mallard, eds, nist chemistry webbook, nist standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen. Standard Enthalpy Of Formation Of H2.
From www.youtube.com
Enthalpies of Reactions Using Average Bond Enthalpies Chemistry Standard Enthalpy Of Formation Of H2 When would one use it in a calculation of enthalpy change? The standard enthalpy of formation of any element in its standard state is zero by definition. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these. Standard Enthalpy Of Formation Of H2.
From www.youtube.com
Enthalpies of Formation Chemsitry Tutorial YouTube Standard Enthalpy Of Formation Of H2 A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. When would one use it in a calculation of enthalpy change? 193 rows. Standard Enthalpy Of Formation Of H2.
From studylib.net
Standard Enthalpy of Formation and Reaction Standard Enthalpy Of Formation Of H2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element in its standard. Standard Enthalpy Of Formation Of H2.
From www.chegg.com
Solved Use the standard enthalpies of formation in the table Standard Enthalpy Of Formation Of H2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. All standard state, 25 °c and 1 bar (written to 1 decimal place). Mallard, eds, nist chemistry webbook, nist standard. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in. Standard Enthalpy Of Formation Of H2.
From www.tessshebaylo.com
Balance The Following Chemical Equation And Calculate Standard Enthalpy Standard Enthalpy Of Formation Of H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation of any element in its standard state is zero by definition. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. A standard enthalpy of formation $δh°_f$ is an enthalpy change. Standard Enthalpy Of Formation Of H2.
From www.slideshare.net
Tang 03 enthalpy of formation and combustion Standard Enthalpy Of Formation Of H2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. When would one use it in a calculation of enthalpy change? The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. 193 rows in. Standard Enthalpy Of Formation Of H2.
From stahonorschemistry.weebly.com
III Calculating Enthalpies STA Form IV Honors Chemistry Standard Enthalpy Of Formation Of H2 A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Mallard, eds, nist chemistry webbook, nist standard. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation of any element in its standard state is zero. Standard Enthalpy Of Formation Of H2.
From www.youtube.com
CHEMISTRY 101 Standard Enthalpy of reaction from Standard Enthalpies Standard Enthalpy Of Formation Of H2 The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. All standard state, 25 °c and 1 bar (written to 1 decimal place). For. Standard Enthalpy Of Formation Of H2.
From www.youtube.com
CHEMISTRY 101 Standard enthalpies of formation and reaction YouTube Standard Enthalpy Of Formation Of H2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. The standard enthalpy of formation. Standard Enthalpy Of Formation Of H2.
From askfilo.com
The standard enthalpies of formation of C2 H5 OH(1),CO2 ( g) and H2 O(1) Standard Enthalpy Of Formation Of H2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. Standard enthalpy change of formation (data table) these tables include heat of formation. Standard Enthalpy Of Formation Of H2.
From www.coursehero.com
[Solved] Use standard enthalpies of formation to calculate the enthalpy Standard Enthalpy Of Formation Of H2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free.. Standard Enthalpy Of Formation Of H2.
From www.nagwa.com
Question Video Calculating the Standard Heat of Reaction for the Standard Enthalpy Of Formation Of H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Mallard, eds, nist chemistry webbook, nist standard. When would one use it in a calculation of enthalpy change? Standard enthalpy change of. Standard Enthalpy Of Formation Of H2.
From www.numerade.com
SOLVED Calculate the standard enthalpy of formation of reaction 2H2(g Standard Enthalpy Of Formation Of H2 For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Mallard, eds, nist. Standard Enthalpy Of Formation Of H2.
From www.slideserve.com
PPT THERMOCHEMISTRY PowerPoint Presentation, free download ID5773812 Standard Enthalpy Of Formation Of H2 Mallard, eds, nist chemistry webbook, nist standard. All standard state, 25 °c and 1 bar (written to 1 decimal place). When would one use it in a calculation of enthalpy change? For example, although oxygen can exist as ozone (o 3), atomic oxygen (o),. The standard enthalpy of formation is defined as the change in enthalpy when one mole of. Standard Enthalpy Of Formation Of H2.
From lessonluft.z19.web.core.windows.net
Heat Of Formation Chart Standard Enthalpy Of Formation Of H2 Mallard, eds, nist chemistry webbook, nist standard. When would one use it in a calculation of enthalpy change? 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard. Standard Enthalpy Of Formation Of H2.
From www.studocu.com
Standard Enthalpy OF Formation C 2 H 2 (g) +226 H 2 SO 4 (l) −811 Standard Enthalpy Of Formation Of H2 Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. A standard enthalpy of formation $δh°_f$ is an enthalpy change for a reaction in which exactly 1 mole of a pure substance is formed from free. When would one use it in a calculation of enthalpy change? The standard. Standard Enthalpy Of Formation Of H2.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Of H2 All standard state, 25 °c and 1 bar (written to 1 decimal place). 193 rows in chemistry and thermodynamics, the standard enthalpy of formation or standard heat of formation of a compound is the change of. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy. Standard Enthalpy Of Formation Of H2.
From brunofuga.adv.br
Standard Enthalpy Of Formation Definition, Table, Equation, 46 OFF Standard Enthalpy Of Formation Of H2 The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. Mallard, eds, nist chemistry webbook, nist standard. Standard enthalpy change of formation (data table) these tables include heat of formation data gathered from a variety of sources, including. When would one use it in a. Standard Enthalpy Of Formation Of H2.
From general.chemistrysteps.com
Standard Enthalpies of Formation Chemistry Steps Standard Enthalpy Of Formation Of H2 The standard enthalpy of formation of any element in its standard state is zero by definition. Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. All standard state, 25 °c and 1 bar (written to 1 decimal place). From what i understand hydrogen only exists as a monatomic. Standard Enthalpy Of Formation Of H2.
From www.slideserve.com
PPT Enthalpy of Formation PowerPoint Presentation, free download ID Standard Enthalpy Of Formation Of H2 Definition and explanation of the terms standard state and standard enthalpy of formation, with listing of values for standard enthalpy and. The standard enthalpy of formation is defined as the change in enthalpy when one mole of a substance in the standard state (1 atm of. From what i understand hydrogen only exists as a monatomic gas at very high. Standard Enthalpy Of Formation Of H2.