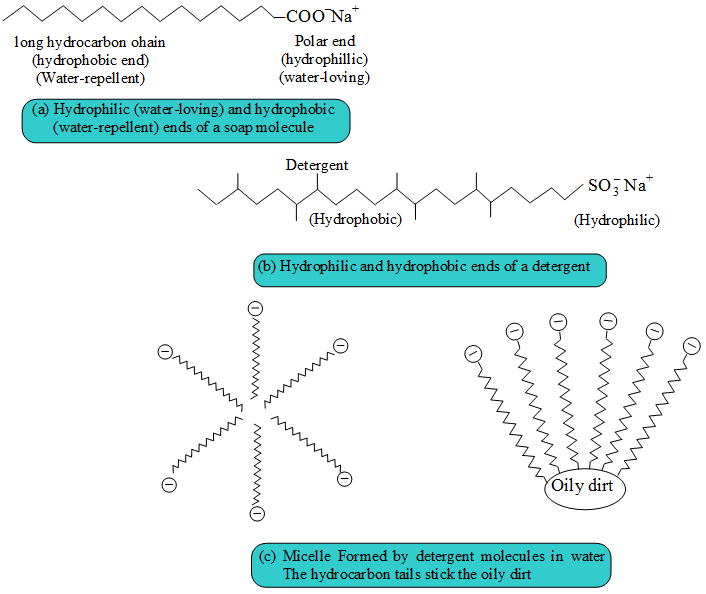
Soap And Detergent Group . The most common examples of such compounds are soaps and detergents, four of which are shown below. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these molecules has a. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. Detergents are also known as surfactants as they have the ability to. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food.
from www.aplustopper.com
Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Detergents are also known as surfactants as they have the ability to. The most common examples of such compounds are soaps and detergents, four of which are shown below. The most common examples of such compounds are soaps and detergents, four of which are shown below. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Note that each of these molecules has a.
Explain the Cleansing Action Of Soaps and Detergents A Plus Topper
Soap And Detergent Group The most common examples of such compounds are soaps and detergents, four of which are shown below. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Note that each of these molecules has a. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The most common examples of such compounds are soaps and detergents, four of which are shown below. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soap And Detergent Group The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Note that each of these molecules has a. Today, detergents. Soap And Detergent Group.
From www.vedantu.com
Soaps and Detergents Classification and Application of Detergents Soap And Detergent Group Note that each of these molecules has a. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the. Soap And Detergent Group.
From studylib.net
Soaps and Other Detergents Soap And Detergent Group Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food.. Soap And Detergent Group.
From www.aplustopper.com
Explain the Cleansing Action Of Soaps and Detergents A Plus Topper Soap And Detergent Group Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these molecules has a. The charged hydrophilic group is also called the head. Soap And Detergent Group.
From aranieco.com
How are soaps different from detergents? Arani Ecosteps Soap And Detergent Group Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're.. Soap And Detergent Group.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID3090261 Soap And Detergent Group The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Detergents are also known as surfactants as they have the ability to. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. Note that each. Soap And Detergent Group.
From www.youtube.com
3. 12C16.4 CT 1 Soaps and Synthetic Detergents YouTube Soap And Detergent Group The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Note that each of these molecules has a nonpolar hydrocarbon. Soap And Detergent Group.
From nearbayou.com
Soap Vs Detergent What is the difference? NearBayou Soap And Detergent Group Note that each of these molecules has a. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the. Soap And Detergent Group.
From studylib.net
THE SCIENCE OF SOAPS AND DETERGENTS Soap And Detergent Group Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Detergents are also known as surfactants as they have the ability to. The most common examples of such compounds are soaps. Soap And Detergent Group.
From allaboutdetergents.com
Detergents and Soap What's the Difference? All About Detergents Soap And Detergent Group Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The most common examples of such compounds are soaps and detergents, four of which are shown below. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. The most common examples of such compounds are soaps and detergents,. Soap And Detergent Group.
From www.youtube.com
Soap and Detergent Manufacturing Business StartupYo www.startupyo Soap And Detergent Group Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Note that each of these molecules has a. Detergents are also known as surfactants as they have the ability to. The most common examples of such compounds are. Soap And Detergent Group.
From richkosh.blogspot.com
EXAMS AND ME Soaps and Detergents Soap And Detergent Group Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these molecules has a. The charged hydrophilic group is also called the head. Soap And Detergent Group.
From www.slideserve.com
PPT Soaps & Detergents PowerPoint Presentation, free download ID Soap And Detergent Group Detergents are also known as surfactants as they have the ability to. The most common examples of such compounds are soaps and detergents, four of which are shown below. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Note that each of these molecules has a nonpolar hydrocarbon chain, the. Soap And Detergent Group.
From www.slideserve.com
PPT Soap and Detergents Manufacture PowerPoint Presentation, free Soap And Detergent Group Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food.. Soap And Detergent Group.
From www.elucidate.org.au
Soaps and Detergents What is the main chemical process involved in Soap And Detergent Group The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The most common examples of such compounds are soaps and. Soap And Detergent Group.
From www.homesciencetools.com
Properties of Soaps & Detergents, Small Group Aldon Soap And Detergent Group Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Detergents are also known as surfactants as they have the ability to. Revise the action. Soap And Detergent Group.
From www.slideserve.com
PPT Soap and Detergents Manufacture PowerPoint Presentation, free Soap And Detergent Group The most common examples of such compounds are soaps and detergents, four of which are shown below. Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents. Soap And Detergent Group.
From aliceinchemiland.blogspot.com
Organic Chemistry in My Daily Life Organic Chemistry about Soap and Soap And Detergent Group The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our. Soap And Detergent Group.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation ID1978873 Soap And Detergent Group Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The most common examples of such compounds are soaps and detergents, four of. Soap And Detergent Group.
From www.slideserve.com
PPT Soap and Detergents Manufacture PowerPoint Presentation, free Soap And Detergent Group Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. Detergents are also known as surfactants as they have the ability to. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of these molecules has a. Revise the action of soaps and detergents for higher chemistry,. Soap And Detergent Group.
From www.youtube.com
Difference between Soap and Detergent YouTube Soap And Detergent Group The most common examples of such compounds are soaps and detergents, four of which are shown below. Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Detergents are also known as surfactants as they have the ability to. Embark on a journey to. Soap And Detergent Group.
From brainly.in
what is the difference between the molecules of soap and detergents Soap And Detergent Group Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Note that each of these molecules has a. The charged hydrophilic group is. Soap And Detergent Group.
From www.yourbestdigs.com
The Best Laundry Detergent of 2020 [Real Testing] Soap And Detergent Group Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The most common examples of such compounds are soaps and detergents, four of which are shown below. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives. Soap And Detergent Group.
From industrytechnologyreports.home.blog
Soap & Detergent Market Worth 207.56 Billion By 2025 Market Research Soap And Detergent Group Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. The charged hydrophilic group is. Soap And Detergent Group.
From www.slideshare.net
Soap and detergent Soap And Detergent Group Detergents are also known as surfactants as they have the ability to. Note that each of these molecules has a. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food.. Soap And Detergent Group.
From studylib.net
SOAPS AND DETERGENTS Soap And Detergent Group Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Detergents are also known as surfactants as they have the ability to. Note. Soap And Detergent Group.
From monitoringclub.org
3 Differences Between Soap Vs Detergent » 2024 Soap And Detergent Group Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The most common examples of such compounds are soaps and detergents, four of which are shown below. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Detergents are also known as surfactants as they have. Soap And Detergent Group.
From www.slideserve.com
PPT SOAPS AND DETERGENTS PowerPoint Presentation, free download ID Soap And Detergent Group Note that each of these molecules has a. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. Today, detergents. Soap And Detergent Group.
From childhealthpolicy.vumc.org
🐈 Soaps and detergents are. What are Soaps and Detergents?. 20221102 Soap And Detergent Group Today, detergents are more likely to be a mixture of synthetic chemicals and additives cooked up in a huge chemical plant and, unlike traditional soap, they're. Note that each of these molecules has a nonpolar hydrocarbon chain, the tail,. The most common examples of such compounds are soaps and detergents, four of which are shown below. Note that each of. Soap And Detergent Group.
From www.youtube.com
What is Saponification? Structure and Action of Soaps and Detergents Soap And Detergent Group Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Note that each of these molecules has a. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. Detergents are also known as surfactants as they have the ability to.. Soap And Detergent Group.
From www.yourbestdigs.com
The Best Laundry Detergents of 2024 Reviews by Your Best Digs Soap And Detergent Group The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail. The most common examples of such compounds are soaps and detergents, four of which are shown below. Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and. Soap And Detergent Group.
From www.slideshare.net
Soap and detergent Soap And Detergent Group Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. The most common examples of such compounds are soaps and. Soap And Detergent Group.
From www.youtube.com
Soaps And Detergents Detailed Explanations YouTube Soap And Detergent Group Note that each of these molecules has a. The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. The most. Soap And Detergent Group.
From tidydiary.com
What’s The Difference Between Laundry Soap And Detergent? Tidy Diary Soap And Detergent Group Note that each of these molecules has a. Revise the action of soaps and detergents for higher chemistry, and learn about the important role of emulsifiers in our food. Detergents are also known as surfactants as they have the ability to. The charged hydrophilic group is also called the head and the long lipophilic hydrocarbon group is called the tail.. Soap And Detergent Group.
From top10gears.com
Types of Laundry Detergents Soap And Detergent Group Embark on a journey to uncover the fascinating world of soaps and detergents, the essential cleaning agents that make our everyday lives cleaner and more hygienic. The most common examples of such compounds are soaps and detergents, four of which are shown below. Detergents are also known as surfactants as they have the ability to. Note that each of these. Soap And Detergent Group.