Capillary Tubes Phenomena . The rise of water in a thin tube inserted in water is caused by forces of attraction between. Why does ice float in water? We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. Capillarity is the result of surface, or interfacial, forces. It is exploited in a number of biological processes, including.
from www.chegg.com
One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. The rise of water in a thin tube inserted in water is caused by forces of attraction between. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. Why does ice float in water? One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. It is exploited in a number of biological processes, including. Capillarity is the result of surface, or interfacial, forces.
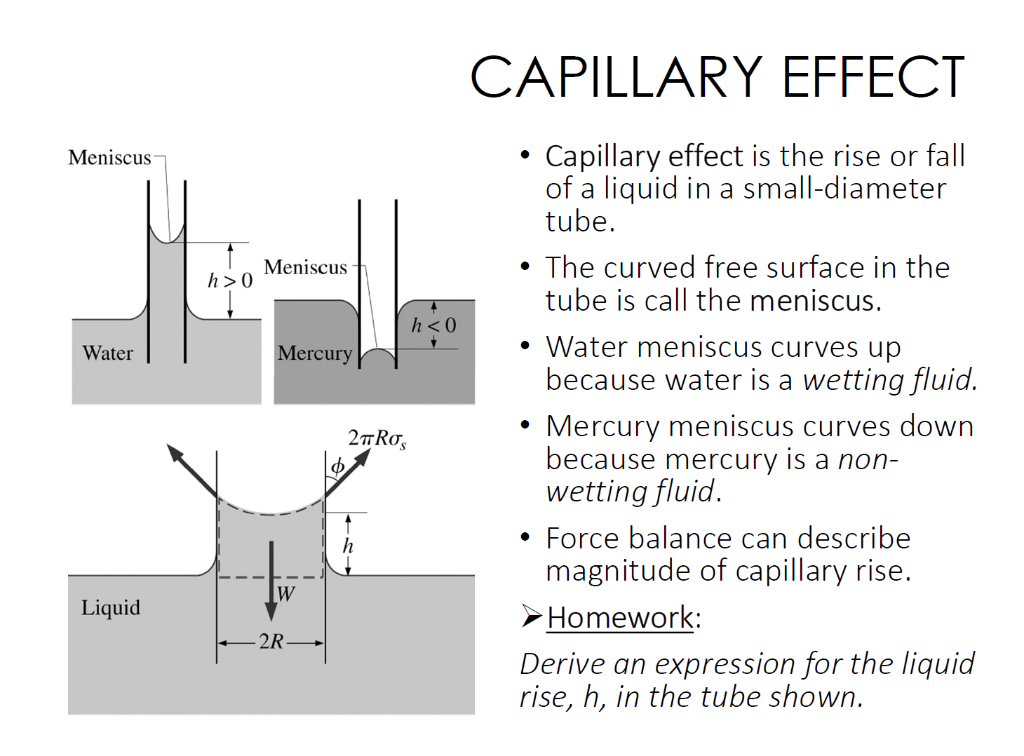
Solved CAPILLARY EFFECT • Capillary effect is the rise or
Capillary Tubes Phenomena The rise of water in a thin tube inserted in water is caused by forces of attraction between. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. The rise of water in a thin tube inserted in water is caused by forces of attraction between. It is exploited in a number of biological processes, including. Capillarity is the result of surface, or interfacial, forces. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. Why does ice float in water?
From www.thoughtco.com
An Illustrated Guide to Capillary Fluid Exchange Capillary Tubes Phenomena We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Why does ice float in water? Capillarity is the result of surface, or interfacial, forces. It is exploited in a number of biological processes, including. Adhesive forces between the molecules of a liquid and different molecules composing a surface in. Capillary Tubes Phenomena.
From www.researchgate.net
(a) Sketch of capillary rise in a vertical tube. (b) Sketch of the Capillary Tubes Phenomena Capillarity is the result of surface, or interfacial, forces. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Why does ice float in water? One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be. Capillary Tubes Phenomena.
From www.ddcoatings.co.uk
What is Capillary Action? Capillary Tubes Phenomena It is exploited in a number of biological processes, including. Capillarity is the result of surface, or interfacial, forces. Why does ice float in water? We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary. Capillary Tubes Phenomena.
From www.researchgate.net
Fluid rise in capillary tube [5]. Download Scientific Diagram Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Why does ice float in water? The rise of water in a thin tube inserted in water is caused by forces of attraction between. We can define capillary action as a phenomenon where the ascension of liquids. Capillary Tubes Phenomena.
From www.physicsforums.com
Infinite flow with capillary tubes? Capillary Tubes Phenomena Why does ice float in water? Capillarity is the result of surface, or interfacial, forces. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. We can define capillary action as a phenomenon where the ascension of liquids through a. Capillary Tubes Phenomena.
From www.youtube.com
Capillary action dissected YouTube Capillary Tubes Phenomena We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Why does ice float in water? Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. One important phenomenon related. Capillary Tubes Phenomena.
From www.chegg.com
Solved CAPILLARY EFFECT • Capillary effect is the rise or Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. The rise of water in a thin tube inserted in water is caused by forces of attraction between. It is exploited in a number of biological processes, including. Capillarity is the result of surface, or interfacial, forces.. Capillary Tubes Phenomena.
From michmet.com
Capillarity and Wetting Phenomena Drops, Bubbles, Pearls, Waves Capillary Tubes Phenomena Capillarity is the result of surface, or interfacial, forces. The rise of water in a thin tube inserted in water is caused by forces of attraction between. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Adhesive forces between the molecules of a liquid and different. Capillary Tubes Phenomena.
From sciencenotes.org
Capillary Action What It Is and How It Works Capillary Tubes Phenomena Capillarity is the result of surface, or interfacial, forces. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. Why does ice float in water? It is exploited in a number of biological processes, including. We can define capillary action. Capillary Tubes Phenomena.
From www.youtube.com
Surface tension and capillary rise YouTube Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary. Capillary Tubes Phenomena.
From www.peoi.org
Chapter 10 Section C Properties of Liquids Capillary Tubes Phenomena It is exploited in a number of biological processes, including. Capillarity is the result of surface, or interfacial, forces. The rise of water in a thin tube inserted in water is caused by forces of attraction between. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be.. Capillary Tubes Phenomena.
From www.researchgate.net
A schematic showing menisci and capillary effect. (a) Two capillary Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. It is exploited in a number of. Capillary Tubes Phenomena.
From web.mit.edu
Capillarity and gravity Capillary Tubes Phenomena Why does ice float in water? Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Capillarity. Capillary Tubes Phenomena.
From brainly.in
what is phenomenon of capillarity? derive an expression for rise of Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Why does ice float in water? One important phenomenon related to the relative strength of cohesive and. Capillary Tubes Phenomena.
From www.meritnation.com
What is the phenomenon of capillarity Derive expression for the rise of Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary. Capillary Tubes Phenomena.
From flatworldknowledge.lardbucket.org
Unique Properties of Liquids Capillary Tubes Phenomena Why does ice float in water? We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. It is exploited. Capillary Tubes Phenomena.
From www.slideserve.com
PPT Chapter 2 Properties of Fluids PowerPoint Presentation, free Capillary Tubes Phenomena Why does ice float in water? It is exploited in a number of biological processes, including. The rise of water in a thin tube inserted in water is caused by forces of attraction between. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Adhesive forces between. Capillary Tubes Phenomena.
From www.researchgate.net
Selfsiphon and siphon diode. (A) Bending the straight peristomemimetic Capillary Tubes Phenomena Capillarity is the result of surface, or interfacial, forces. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. The rise of water in a thin tube inserted in water is caused by forces of attraction between. Why does ice float in water? Adhesive forces between the. Capillary Tubes Phenomena.
From ar.inspiredpencil.com
Capillary Action Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. The rise of water in a thin tube inserted in water is caused by forces of attraction between. Capillarity is the result of surface, or interfacial, forces. Adhesive forces between the molecules of a liquid and different. Capillary Tubes Phenomena.
From www.vedantu.com
When a capillary tube is immersed vertically in water class 11 physics Capillary Tubes Phenomena It is exploited in a number of biological processes, including. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Why does ice float in water? One. Capillary Tubes Phenomena.
From www.mechdoctor.com
What is Capillarity? REFINERY OIL AND GAS Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Capillarity is the result of surface, or interfacial, forces. The rise of water in a thin tube inserted in water is caused by forces of attraction between. One important phenomenon related to the relative strength of cohesive. Capillary Tubes Phenomena.
From www.researchgate.net
The capillary tubes are filled with a colloidal suspension. The single Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary. Capillary Tubes Phenomena.
From interfacegroup.ch
Capillary effects from garden to lab applications The Interface Group Capillary Tubes Phenomena Why does ice float in water? One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Capillarity is the result of surface, or interfacial, forces. It is exploited in a number of biological processes, including. One important phenomenon related to the relative strength of cohesive and adhesive. Capillary Tubes Phenomena.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. We can define capillary action as. Capillary Tubes Phenomena.
From www.alamy.com
Capillary Attraction Repulsion (PSF Stock Photo Alamy Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. Capillarity is the result of surface,. Capillary Tubes Phenomena.
From courses.lumenlearning.com
Cohesion and Adhesion in Liquids Surface Tension and Capillary Action Capillary Tubes Phenomena One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. It is exploited in a number of biological processes, including. We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes. Capillary Tubes Phenomena.
From www.researchgate.net
Mechanism of inner microstructures enhancing capillary rise in tubes Capillary Tubes Phenomena Capillarity is the result of surface, or interfacial, forces. The rise of water in a thin tube inserted in water is caused by forces of attraction between. Why does ice float in water? One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a. Capillary Tubes Phenomena.
From www.researchgate.net
Capillary Tubes Showing Air Water Interface at Varying Heights for Capillary Tubes Phenomena The rise of water in a thin tube inserted in water is caused by forces of attraction between. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. We can define capillary action as a phenomenon where the ascension. Capillary Tubes Phenomena.
From lewis-has-ritter.blogspot.com
Capillary Action Is a Term Used to Describe How LewishasRitter Capillary Tubes Phenomena Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. It is exploited in a number of biological processes, including. Capillarity is the result of surface, or interfacial, forces. We can define capillary action as a phenomenon where the ascension. Capillary Tubes Phenomena.
From www.youtube.com
Capillarity_Capillary Rise & Fall YouTube Capillary Tubes Phenomena We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. Why does ice float in water? Capillarity is the result. Capillary Tubes Phenomena.
From www.researchgate.net
Rise of water in a capillary tube. On the right side the contact angle Capillary Tubes Phenomena We can define capillary action as a phenomenon where the ascension of liquids through a tube or cylinder takes place. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action —the tendency of a fluid to be. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with. Capillary Tubes Phenomena.
From www.biolinscientific.com
Capillary action how contact angle and surface tension are related? Capillary Tubes Phenomena Why does ice float in water? One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. Capillarity is the result of surface, or interfacial, forces. Adhesive forces between the molecules of a liquid and different molecules composing a surface. Capillary Tubes Phenomena.
From www.quirkyscience.com
Capillary Action from the Forces of Adhesion and Cohesion Capillary Tubes Phenomena The rise of water in a thin tube inserted in water is caused by forces of attraction between. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. Why does ice float in water? Capillarity is the result of surface,. Capillary Tubes Phenomena.
From www.researchgate.net
Capillary rise dynamics and quantification. (A) Optical image of water Capillary Tubes Phenomena Why does ice float in water? One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid to be raised or suppressed in a narrow tube, or capillary. The rise of water in a thin tube inserted in water is caused by forces of attraction between. We can define capillary action. Capillary Tubes Phenomena.
From www.researchgate.net
a Representative photographs of capillary tubes with remarkable scale Capillary Tubes Phenomena It is exploited in a number of biological processes, including. Adhesive forces between the molecules of a liquid and different molecules composing a surface in contact with the liquid are responsible for phenomena such as surface wetting and capillary rise. One important phenomenon related to the relative strength of cohesive and adhesive forces is capillary action—the tendency of a fluid. Capillary Tubes Phenomena.