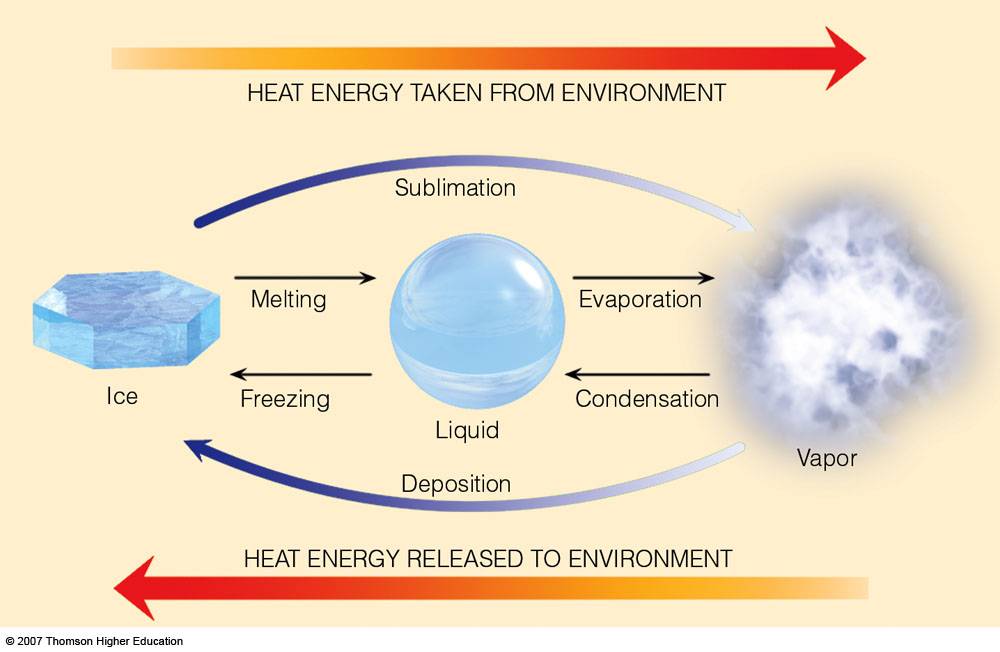
What Is The Enthalpy Of Fusion Of Ice . The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. Learning how to calculate the heat of fusion is fairly straightforward; It is sometimes called enthalpy of fusion or latent heat of fusion. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. It's also known as enthalpy of fusion. This process is better known as melting, or heat of fusion, and results. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. The most common example is solid ice turning into liquid water. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Its units are usually joules per gram (j/g) or calories per gram (cal/g).
from apollo.lsc.vsc.edu
It's also known as enthalpy of fusion. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. It is sometimes called enthalpy of fusion or latent heat of fusion. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). This process is better known as melting, or heat of fusion, and results. The most common example is solid ice turning into liquid water. Learning how to calculate the heat of fusion is fairly straightforward;
Latent Heats sublimation and deposition
What Is The Enthalpy Of Fusion Of Ice The most common example is solid ice turning into liquid water. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. It is sometimes called enthalpy of fusion or latent heat of fusion. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. The most common example is solid ice turning into liquid water. It's also known as enthalpy of fusion. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. Learning how to calculate the heat of fusion is fairly straightforward; This process is better known as melting, or heat of fusion, and results.
From chemistnotes.com
Enthalpy Definition, expression, types Chemistry Notes What Is The Enthalpy Of Fusion Of Ice Learning how to calculate the heat of fusion is fairly straightforward; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Its units are usually joules per gram (j/g) or calories per gram (cal/g). This example problem demonstrates how to. What Is The Enthalpy Of Fusion Of Ice.
From brainly.in
draw labelled diagram of the experimental set up to the latent heat of fusion of ice Brainly.in What Is The Enthalpy Of Fusion Of Ice Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. This process is better known as melting, or heat of fusion, and results. It's also known as enthalpy of fusion. In the context of ice, the enthalpy of fusion generally. What Is The Enthalpy Of Fusion Of Ice.
From www.coursehero.com
[Solved] Using your results for Experiment 3 Measuring the Enthalpy of... Course Hero What Is The Enthalpy Of Fusion Of Ice In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. Its units are usually joules per gram (j/g) or calories per gram (cal/g). The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted. What Is The Enthalpy Of Fusion Of Ice.
From www.youtube.com
Latent Heat of Fusion of Ice by an Experiment, Physics Lecture Sabaq.pk YouTube What Is The Enthalpy Of Fusion Of Ice The most common example is solid ice turning into liquid water. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. Learning how to calculate the heat of fusion is fairly straightforward; Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is. What Is The Enthalpy Of Fusion Of Ice.
From www.chegg.com
Solved G15 Part II. Enthalpy of fusion of ice your graph What Is The Enthalpy Of Fusion Of Ice This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). It is sometimes called enthalpy of fusion or latent heat of fusion. This process is better known as melting, or heat of fusion, and results. The heat which. What Is The Enthalpy Of Fusion Of Ice.
From chemistrytalk.org
Heat of Fusion Explained ChemTalk What Is The Enthalpy Of Fusion Of Ice It's also known as enthalpy of fusion. The most common example is solid ice turning into liquid water. Its units are usually joules per gram (j/g) or calories per gram (cal/g). The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Learning how to. What Is The Enthalpy Of Fusion Of Ice.
From www.youtube.com
Calculating Heat of fusion YouTube What Is The Enthalpy Of Fusion Of Ice The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. Heat of fusion, also called enthalpy. What Is The Enthalpy Of Fusion Of Ice.
From www.chegg.com
Quantitative study of the entalpy of fusion of ice. What Is The Enthalpy Of Fusion Of Ice The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. It is sometimes called enthalpy of fusion or. What Is The Enthalpy Of Fusion Of Ice.
From www.youtube.com
Calculate the enthalpy change on freezing of 1.0mol of water at 10.0oC to ice at 10.0oC. YouTube What Is The Enthalpy Of Fusion Of Ice This process is better known as melting, or heat of fusion, and results. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted. What Is The Enthalpy Of Fusion Of Ice.
From www.slideserve.com
PPT Heat of Fusion PowerPoint Presentation, free download ID2249917 What Is The Enthalpy Of Fusion Of Ice The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. In the context of. What Is The Enthalpy Of Fusion Of Ice.
From www.tec-science.com
Specific latent heat of fusion (enthalpy of fusion) tecscience What Is The Enthalpy Of Fusion Of Ice This process is better known as melting, or heat of fusion, and results. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. It's also known as enthalpy of fusion. This example problem demonstrates how to calculate the amount of energy required to melt a sample. What Is The Enthalpy Of Fusion Of Ice.
From www.chegg.com
Solved Background Part 2. Enthalpy of fusion of ice When ice What Is The Enthalpy Of Fusion Of Ice It is sometimes called enthalpy of fusion or latent heat of fusion. The most common example is solid ice turning into liquid water. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. In the context of ice, the enthalpy. What Is The Enthalpy Of Fusion Of Ice.
From www.studypool.com
SOLUTION Enthalpy Of Fusion Of Ice Studypool What Is The Enthalpy Of Fusion Of Ice The most common example is solid ice turning into liquid water. Its units are usually joules per gram (j/g) or calories per gram (cal/g). It is sometimes called enthalpy of fusion or latent heat of fusion. It's also known as enthalpy of fusion. This process is better known as melting, or heat of fusion, and results. The heat of fusion. What Is The Enthalpy Of Fusion Of Ice.
From www.chegg.com
Solved Calculate the enthalpy of fusion of ice using What Is The Enthalpy Of Fusion Of Ice Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. It's also. What Is The Enthalpy Of Fusion Of Ice.
From www.slideshare.net
Energy ch 16 What Is The Enthalpy Of Fusion Of Ice The most common example is solid ice turning into liquid water. Its units are usually joules per gram (j/g) or calories per gram (cal/g). The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. Learning how to calculate the heat of fusion is fairly. What Is The Enthalpy Of Fusion Of Ice.
From www.pinterest.com
Heat Of Fusion Easy Science Thermodynamics, Chemical changes, Teaching chemistry What Is The Enthalpy Of Fusion Of Ice It's also known as enthalpy of fusion. This process is better known as melting, or heat of fusion, and results. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt. What Is The Enthalpy Of Fusion Of Ice.
From www.youtube.com
Latent heat of fusion of ice YouTube What Is The Enthalpy Of Fusion Of Ice In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. The heat which a solid absorbs when it. What Is The Enthalpy Of Fusion Of Ice.
From www.slideserve.com
PPT Phase Changes and their Calculations PowerPoint Presentation, free download ID3207053 What Is The Enthalpy Of Fusion Of Ice In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Its units are usually joules per. What Is The Enthalpy Of Fusion Of Ice.
From www.slideserve.com
PPT Chapter 17 Changes of Phase PowerPoint Presentation, free download ID373295 What Is The Enthalpy Of Fusion Of Ice It is sometimes called enthalpy of fusion or latent heat of fusion. Its units are usually joules per gram (j/g) or calories per gram (cal/g). This process is better known as melting, or heat of fusion, and results. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or. What Is The Enthalpy Of Fusion Of Ice.
From www.chegg.com
Solved Background Part 2. Enthalpy of fusion of ice When ice What Is The Enthalpy Of Fusion Of Ice Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. It's also known as enthalpy of fusion. The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at. What Is The Enthalpy Of Fusion Of Ice.
From www.youtube.com
Enthalpy of fusion YouTube What Is The Enthalpy Of Fusion Of Ice The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. The most common example is solid ice turning into liquid water. Learning how to calculate the heat of fusion is fairly straightforward; It's also known as enthalpy of fusion. Heat of fusion,. What Is The Enthalpy Of Fusion Of Ice.
From sciencing.com
How to Measure Heat of Fusion of Ice Sciencing What Is The Enthalpy Of Fusion Of Ice In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. It's also known as enthalpy of fusion. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant. What Is The Enthalpy Of Fusion Of Ice.
From www.slideserve.com
PPT Phases of Matter and Solutions PowerPoint Presentation, free download ID2250225 What Is The Enthalpy Of Fusion Of Ice The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. This process is better known as melting, or heat of fusion, and results. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice.. What Is The Enthalpy Of Fusion Of Ice.
From www.ck12.org
Heat of fusion Overview ( Video ) Chemistry CK12 Foundation What Is The Enthalpy Of Fusion Of Ice The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. Learning how. What Is The Enthalpy Of Fusion Of Ice.
From www.youtube.com
Enthalpy of fusion of ice experiment YouTube What Is The Enthalpy Of Fusion Of Ice The most common example is solid ice turning into liquid water. It is sometimes called enthalpy of fusion or latent heat of fusion. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. This process is better known as melting,. What Is The Enthalpy Of Fusion Of Ice.
From www.youtube.com
Pre Lab Heat of Fusion for Ice YouTube What Is The Enthalpy Of Fusion Of Ice This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. Its units are usually joules per gram (j/g) or calories per gram (cal/g). The most common example is solid ice turning into liquid water. This process is better known as melting, or heat of fusion, and results. The heat which a. What Is The Enthalpy Of Fusion Of Ice.
From www.toppr.com
(b) (i) Calculate the entropy change during the melting of one mole of ice into water 0°C What Is The Enthalpy Of Fusion Of Ice The most common example is solid ice turning into liquid water. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. It's also known as enthalpy of fusion. It is sometimes called enthalpy of fusion or latent heat of fusion. Heat of fusion, also called enthalpy of fusion or latent heat. What Is The Enthalpy Of Fusion Of Ice.
From www.slideshare.net
Energy ch 16 What Is The Enthalpy Of Fusion Of Ice It is sometimes called enthalpy of fusion or latent heat of fusion. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of energy needed to melt or freeze a substance under conditions of constant pressure. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water. What Is The Enthalpy Of Fusion Of Ice.
From apollo.lsc.vsc.edu
Latent Heats sublimation and deposition What Is The Enthalpy Of Fusion Of Ice Learning how to calculate the heat of fusion is fairly straightforward; The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is. What Is The Enthalpy Of Fusion Of Ice.
From ruairilucja.blogspot.com
24+ Calculating Heat Of Fusion RuairiLucja What Is The Enthalpy Of Fusion Of Ice The most common example is solid ice turning into liquid water. It's also known as enthalpy of fusion. It is sometimes called enthalpy of fusion or latent heat of fusion. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. Learning how to calculate the heat. What Is The Enthalpy Of Fusion Of Ice.
From www.researchgate.net
Enthalpy of ice from 0 to 273 K, liquid water at 273 K, and empirical... Download Scientific What Is The Enthalpy Of Fusion Of Ice This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. Heat of fusion, also called enthalpy of fusion or latent heat of fusion, is a quantity of. What Is The Enthalpy Of Fusion Of Ice.
From www.studypool.com
SOLUTION Enthalpy Of Fusion Of Ice Studypool What Is The Enthalpy Of Fusion Of Ice This process is better known as melting, or heat of fusion, and results. The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to. What Is The Enthalpy Of Fusion Of Ice.
From studylib.net
2.5(a) Enthalpy What Is The Enthalpy Of Fusion Of Ice It is sometimes called enthalpy of fusion or latent heat of fusion. The heat of fusion is the enthalpy change when a unit mass of a substance changes its state from solid to liquid at a constant temperature and pressure. The most common example is solid ice turning into liquid water. Its units are usually joules per gram (j/g) or. What Is The Enthalpy Of Fusion Of Ice.
From www.researchgate.net
14 gives the enthalpy value of the mixture when entering the ice... Download Scientific Diagram What Is The Enthalpy Of Fusion Of Ice Learning how to calculate the heat of fusion is fairly straightforward; In the context of ice, the enthalpy of fusion generally refers to the energy required to convert ice into water whilst maintaining a constant. It is sometimes called enthalpy of fusion or latent heat of fusion. The heat which a solid absorbs when it melts is called the enthalpy. What Is The Enthalpy Of Fusion Of Ice.
From www.doubtnut.com
Calculate the entropy change in the melting of 1 kg of ice at 0^()C in SI units. Heat of What Is The Enthalpy Of Fusion Of Ice Its units are usually joules per gram (j/g) or calories per gram (cal/g). The heat which a solid absorbs when it melts is called the enthalpy of fusion or heat of fusion and is usually quoted on a molar. This example problem demonstrates how to calculate the amount of energy required to melt a sample of water ice. The heat. What Is The Enthalpy Of Fusion Of Ice.