How Do Electrons Get Their Energy . As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. Electrons can jump from a lower to the next higher energy level if. It's always being pulled closer. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. An electron orbiting a nucleus is electrically attracted to the nucleus; So there is no need to spend energy to keep on moving,. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that. But the electron also has kinetic energy, which works to send the electron flying away. Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. According to bohr's theory, electrons of an atom. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. When an electron gains extra energy, it can become excited. The velocity of a body remains constant unless the body is acted upon by an external force. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition.
from www.nagwa.com
According to bohr's theory, electrons of an atom. It's always being pulled closer. When an electron gains extra energy, it can become excited. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. An electron orbiting a nucleus is electrically attracted to the nucleus; The velocity of a body remains constant unless the body is acted upon by an external force. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. But the electron also has kinetic energy, which works to send the electron flying away. Electrons can jump from a lower to the next higher energy level if. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that.
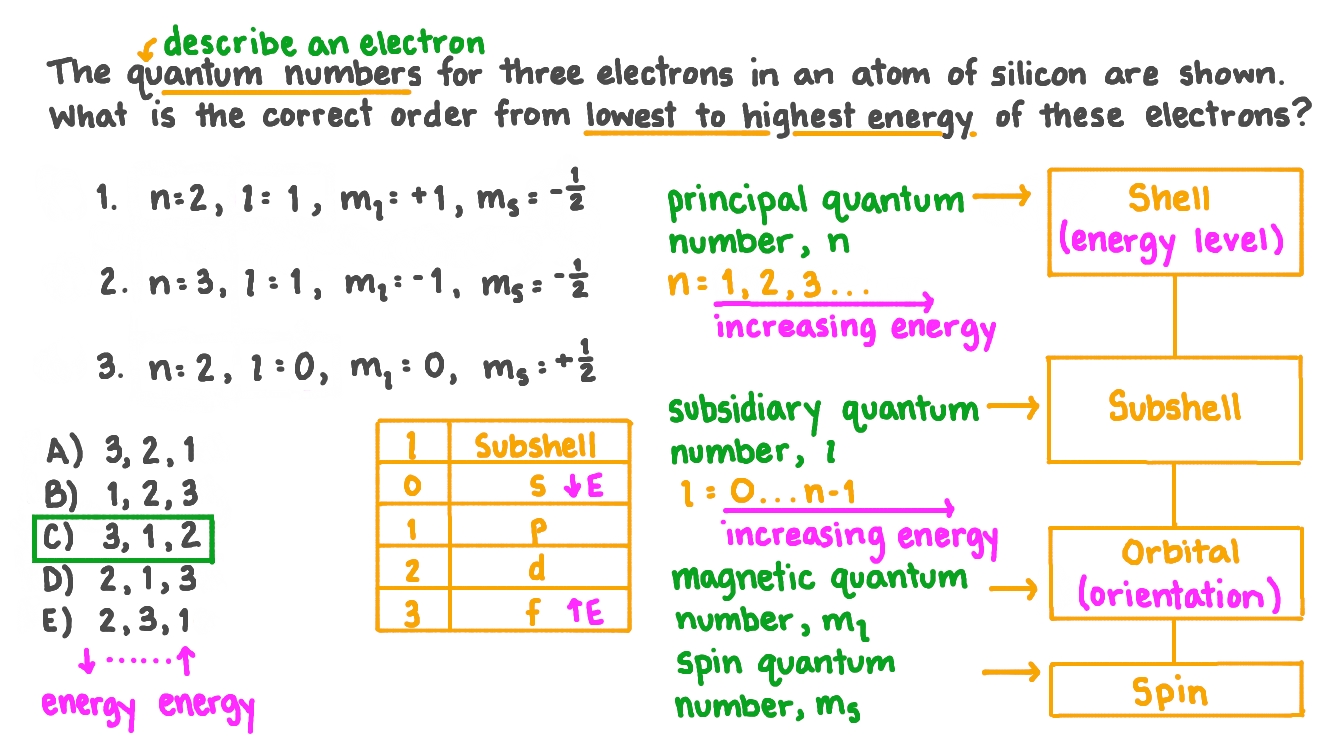
Question Video Ranking Three Electrons from Lowest to Highest Energy
How Do Electrons Get Their Energy Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. It's always being pulled closer. The velocity of a body remains constant unless the body is acted upon by an external force. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. But the electron also has kinetic energy, which works to send the electron flying away. Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. So there is no need to spend energy to keep on moving,. According to bohr's theory, electrons of an atom. When an electron gains extra energy, it can become excited. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. An electron orbiting a nucleus is electrically attracted to the nucleus; Electrons can jump from a lower to the next higher energy level if. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that.
From www.sciencefacts.net
Neutron Definition, Characteristics, & Location with Example How Do Electrons Get Their Energy Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. When an electron gains extra energy, it can become excited. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete. How Do Electrons Get Their Energy.
From www.carlsonstockart.com
Electron Energy Levels of Atoms Carlson Stock Art How Do Electrons Get Their Energy Electrons can jump from a lower to the next higher energy level if. It's always being pulled closer. But the electron also has kinetic energy, which works to send the electron flying away. An electron orbiting a nucleus is electrically attracted to the nucleus; The electron transport chain is a series of protein complexes and electron carrier molecules within the. How Do Electrons Get Their Energy.
From www.nagwa.com
Question Video Identifying the Number of Electrons in the Outermost How Do Electrons Get Their Energy Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. An electron orbiting a nucleus is electrically attracted to the nucleus; So there is no need to spend energy to keep on moving,. When an electron gains extra energy, it can become. How Do Electrons Get Their Energy.
From sciencenotes.org
What Are Valence Electrons? Definition and Periodic Table How Do Electrons Get Their Energy As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. But the electron also has kinetic energy, which works to send the electron flying away. Energy is emitted from the atom when the electron. How Do Electrons Get Their Energy.
From www.sciencefacts.net
Electron Shell Definition & Number of Electrons in Each Shell How Do Electrons Get Their Energy An electron orbiting a nucleus is electrically attracted to the nucleus; Atoms lose electrons because of how they interact with forces beyond atomic nuclei. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that. Shown here is the first balmer transition, in which an electron jumps from orbit n. How Do Electrons Get Their Energy.
From www.expii.com
Valence Electrons — Definition & Importance Expii How Do Electrons Get Their Energy As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. An electron orbiting a nucleus is electrically attracted to the nucleus; According to bohr's theory, electrons of an atom.. How Do Electrons Get Their Energy.
From www.youtube.com
Where Do Electrons Get Energy To Spin? quantum energy space How Do Electrons Get Their Energy Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. According to bohr's theory, electrons of an atom. Electrons can jump from a lower. How Do Electrons Get Their Energy.
From www.secretsofuniverse.in
What Does An Electron's Spin Actually Represent And How Was It How Do Electrons Get Their Energy According to bohr's theory, electrons of an atom. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. It's always being pulled closer. So there is no need to spend energy to keep. How Do Electrons Get Their Energy.
From www.nagwa.com
Lesson Electron Energy Level Transitions Nagwa How Do Electrons Get Their Energy Atoms lose electrons because of how they interact with forces beyond atomic nuclei. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electrons can jump from a lower to the next higher energy level if. In this section we will discuss the energy level of the electron of a hydrogen. How Do Electrons Get Their Energy.
From www.youtube.com
Where do electrons get energy to spin around an atom's nucleus? YouTube How Do Electrons Get Their Energy The velocity of a body remains constant unless the body is acted upon by an external force. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria. How Do Electrons Get Their Energy.
From oncologymedicalphysics.com
Basic Radiation Physics Oncology Medical Physics How Do Electrons Get Their Energy Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Electrons can jump from a lower to the next higher energy level if. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. Every electron ever observed, whether. How Do Electrons Get Their Energy.
From www.youtube.com
Electron Energy Levels and Photons IB Physics YouTube How Do Electrons Get Their Energy Atoms lose electrons because of how they interact with forces beyond atomic nuclei. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes.. How Do Electrons Get Their Energy.
From www.nagwa.com
Question Video Ranking Three Electrons from Lowest to Highest Energy How Do Electrons Get Their Energy The velocity of a body remains constant unless the body is acted upon by an external force. Electrons can jump from a lower to the next higher energy level if. It's always being pulled closer. According to bohr's theory, electrons of an atom. When an electron gains extra energy, it can become excited. As you go farther from the nucleus,. How Do Electrons Get Their Energy.
From www.youtube.com
Where Do Electrons Get Everlasting Energy? YouTube How Do Electrons Get Their Energy It's always being pulled closer. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. The velocity of a body remains constant unless the body is acted upon by an external force.. How Do Electrons Get Their Energy.
From kaylynn-has-mills.blogspot.com
How Many Electrons in the Second Energy Level KaylynnhasMills How Do Electrons Get Their Energy Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes transition. According to bohr's theory, electrons of an. How Do Electrons Get Their Energy.
From www.slideserve.com
PPT Electrons in Atoms PowerPoint Presentation, free download ID How Do Electrons Get Their Energy The velocity of a body remains constant unless the body is acted upon by an external force. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. When an electron gains extra energy, it can become excited. An electron orbiting a nucleus. How Do Electrons Get Their Energy.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica How Do Electrons Get Their Energy But the electron also has kinetic energy, which works to send the electron flying away. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. The velocity of a body remains constant unless the body is acted upon by an external force. Energy is emitted from the atom when the electron jumps from one orbit to another. How Do Electrons Get Their Energy.
From www.sciencesfp.com
Electronic structure of matter. San Francisco de Paula, Science How Do Electrons Get Their Energy As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. So there is no need to spend energy to keep on moving,. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly. How Do Electrons Get Their Energy.
From www.britannica.com
Atom Electrons, Orbitals, Energy Britannica How Do Electrons Get Their Energy Atoms lose electrons because of how they interact with forces beyond atomic nuclei. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by. How Do Electrons Get Their Energy.
From learn.sparkfun.com
What is Electricity? SparkFun Learn How Do Electrons Get Their Energy Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. The velocity of a body remains constant unless the body. How Do Electrons Get Their Energy.
From slidetodoc.com
Essential Question How do electrons get transferred Conduction How Do Electrons Get Their Energy An electron orbiting a nucleus is electrically attracted to the nucleus; Electrons can jump from a lower to the next higher energy level if. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. But the. How Do Electrons Get Their Energy.
From www.youtube.com
Where Do Electrons Get Their Everlasting Energy? YouTube How Do Electrons Get Their Energy Electrons can jump from a lower to the next higher energy level if. According to bohr's theory, electrons of an atom. It's always being pulled closer. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that. Shown here is the first balmer transition, in which an electron jumps from. How Do Electrons Get Their Energy.
From www.teachoo.com
Distribution of Electrons in Different Orbits [with Examples] Teacho How Do Electrons Get Their Energy Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. An electron. How Do Electrons Get Their Energy.
From philschatz.com
Elements and Atoms The Building Blocks of Matter · Anatomy and Physiology How Do Electrons Get Their Energy As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. But the electron also has kinetic energy, which works to send the electron flying away. It's always being. How Do Electrons Get Their Energy.
From spmchemistry.blog.onlinetuition.com.my
Electron Arrangement in Atom SPM Chemistry How Do Electrons Get Their Energy Energy is emitted from the atom when the electron jumps from one orbit to another closer to the nucleus. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. It's always being pulled closer. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron. How Do Electrons Get Their Energy.
From www.slideserve.com
PPT How are electrons arranged? PowerPoint Presentation, free How Do Electrons Get Their Energy But the electron also has kinetic energy, which works to send the electron flying away. The velocity of a body remains constant unless the body is acted upon by an external force. According to bohr's theory, electrons of an atom. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a. How Do Electrons Get Their Energy.
From pijaeducation.com
ATOM, ORBITS AND ENERGY LEVELS » PIJA Education How Do Electrons Get Their Energy But the electron also has kinetic energy, which works to send the electron flying away. It's always being pulled closer. The velocity of a body remains constant unless the body is acted upon by an external force. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. Electrons can jump from a lower to the next higher. How Do Electrons Get Their Energy.
From www.slideserve.com
PPT Electrons in Atoms Electron Configuration PowerPoint How Do Electrons Get Their Energy The velocity of a body remains constant unless the body is acted upon by an external force. Atoms lose electrons because of how they interact with forces beyond atomic nuclei. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. Electrons can jump from a lower to. How Do Electrons Get Their Energy.
From www.teachoo.com
How to find Valency? What are valence electrons? Teachoo How Do Electrons Get Their Energy Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. So there is no need to spend energy to keep on moving,. An electron orbiting a nucleus is electrically attracted to the nucleus; In this section we will discuss the energy level. How Do Electrons Get Their Energy.
From www.animalia-life.club
Electrons In An Atom How Do Electrons Get Their Energy The velocity of a body remains constant unless the body is acted upon by an external force. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. So there is no need to spend energy to keep on moving,. The electron transport chain is a series of. How Do Electrons Get Their Energy.
From www.nagwa.com
Question Video Determining the Maximum Number of Electrons That Can How Do Electrons Get Their Energy According to bohr's theory, electrons of an atom. It's always being pulled closer. An electron orbiting a nucleus is electrically attracted to the nucleus; So there is no need to spend energy to keep on moving,. The electron transport chain is a series of protein complexes and electron carrier molecules within the inner membrane of mitochondria that. Shown here is. How Do Electrons Get Their Energy.
From study.com
Electron Transition Definition, Chart & Examples Lesson How Do Electrons Get Their Energy Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks like it's constantly doing tiny pirouettes. Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. It's always being pulled closer. The electron transport chain is a series of protein. How Do Electrons Get Their Energy.
From zakruti.com
How do cells get their energy (Electron Transport Chain) Crash Course How Do Electrons Get Their Energy The velocity of a body remains constant unless the body is acted upon by an external force. Shown here is the first balmer transition, in which an electron jumps from orbit n = 3 to. According to bohr's theory, electrons of an atom. When an electron gains extra energy, it can become excited. An electron orbiting a nucleus is electrically. How Do Electrons Get Their Energy.
From www.expii.com
Valence Electrons — Definition & Importance Expii How Do Electrons Get Their Energy Atoms lose electrons because of how they interact with forces beyond atomic nuclei. As you go farther from the nucleus, electrons at higher levels have more energy, and their energy increases by a fixed, discrete amount. In this section we will discuss the energy level of the electron of a hydrogen atom, and how it changes as the electron undergoes. How Do Electrons Get Their Energy.
From drewdesnhfletcher.blogspot.com
Where Do Electrons Get Their Energy in the Light Reactions How Do Electrons Get Their Energy Electrons can jump from a lower to the next higher energy level if. The velocity of a body remains constant unless the body is acted upon by an external force. According to bohr's theory, electrons of an atom. Every electron ever observed, whether it's just ambling around a carbon atom in your fingernail or speeding through a particle accelerator, looks. How Do Electrons Get Their Energy.