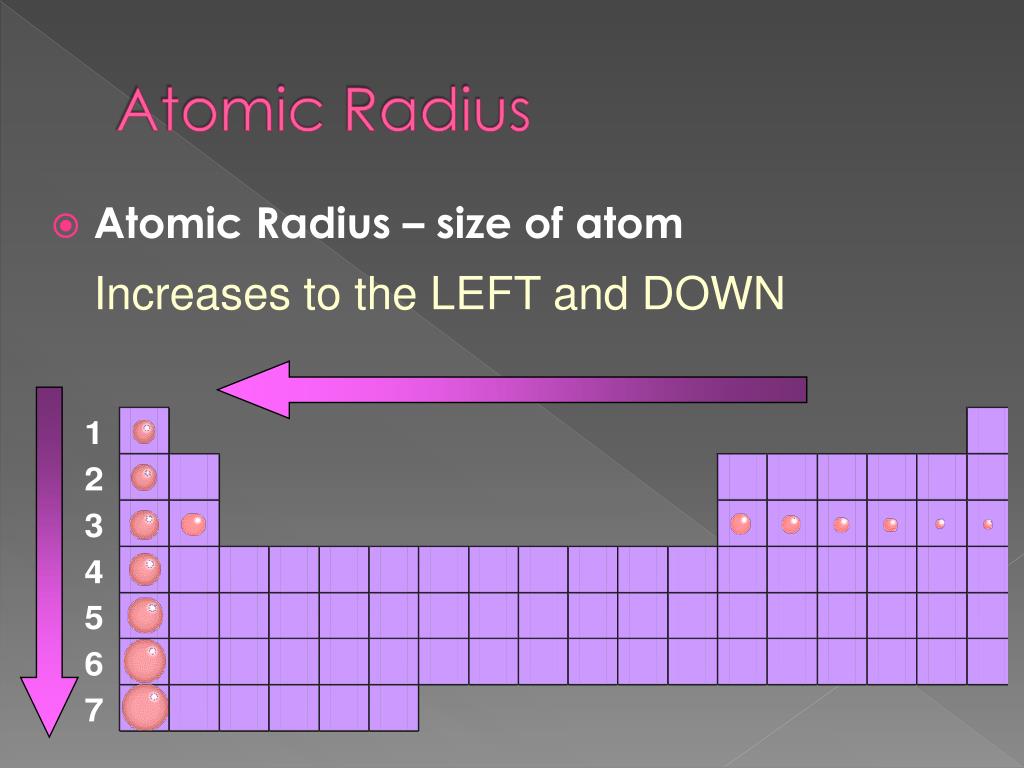
Atomic Radius And Why . Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: Atomic radii can be obtained. Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. This is because atoms gain an electron shell. periodic table trend.
from www.slideserve.com
atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. This is because atoms gain an electron shell. periodic table trend. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic radii can be obtained. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Atomic and ionic radius increase moving down a group or column of the periodic table.
PPT History PowerPoint Presentation, free download ID1587611
Atomic Radius And Why Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. This is because atoms gain an electron shell. periodic table trend. Atomic radii can be obtained. Atomic and ionic radius increase moving down a group or column of the periodic table. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity:
From periodictrendsforhonorschemistry.weebly.com
Atomic Radius Trends of the Periodic Table Atomic Radius And Why atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. This is because atoms gain an electron shell. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each. Atomic Radius And Why.
From www.vrogue.co
Periodic Table Of The Elements Atomic Radius vrogue.co Atomic Radius And Why atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. periodic table trend. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic. Atomic Radius And Why.
From www.slideserve.com
PPT PERIODIC PROPERTIES PowerPoint Presentation, free download ID Atomic Radius And Why Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: periodic table trend. Atomic and ionic radius increase moving down a group or column of the periodic table. This is because atoms gain an electron shell. atomic radius is determined as half the distance between the nuclei of two identical. Atomic Radius And Why.
From www.wisegeek.com
What Is the Atomic Radius? (with pictures) Atomic Radius And Why Atomic radii can be obtained. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: atomic radius is determined as. Atomic Radius And Why.
From www.learnatnoon.com
Atomic size and atomic radius explained Noon Academy Atomic Radius And Why Atomic radii can be obtained. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. the covalent atomic radius (rcov). Atomic Radius And Why.
From www.breakingatom.com
Atomic Radius of Elements Atomic Radius And Why This is because atoms gain an electron shell. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each. Atomic Radius And Why.
From neetlab.com
Atomic Radius Periodic Table NEET Lab Atomic Radius And Why Atomic and ionic radius increase moving down a group or column of the periodic table. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic radii can be obtained. This is because atoms. Atomic Radius And Why.
From www.ck12.org
Atomic Radius Example 2 ( Video ) Chemistry CK12 Foundation Atomic Radius And Why This is because atoms gain an electron shell. Atomic radii can be obtained. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is determined as half the distance between the nuclei of two. Atomic Radius And Why.
From www.sliderbase.com
Periodic Trends Presentation Chemistry Atomic Radius And Why This is because atoms gain an electron shell. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. periodic table trend. Electron configuration determines. Atomic Radius And Why.
From www.slideserve.com
PPT Section 4.5—Periodicity PowerPoint Presentation, free download Atomic Radius And Why Atomic radii can be obtained. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. This is because atoms gain an electron shell. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius. Atomic Radius And Why.
From www.animalia-life.club
Ionic Radius Diagram Atomic Radius And Why Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: This is because atoms gain an electron shell. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. Atomic and ionic. Atomic Radius And Why.
From www.slideserve.com
PPT Chemical Bonding Bonding Theory and Lewis Formulas PowerPoint Atomic Radius And Why atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Atomic radii. Atomic Radius And Why.
From quizgrouchiest.z4.web.core.windows.net
How To Calculate Atomic Radius Atomic Radius And Why This is because atoms gain an electron shell. Atomic radii can be obtained. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. periodic table trend. the covalent atomic radius (rcov) is half the internuclear distance in. Atomic Radius And Why.
From www.chemistrystudent.com
Chemistry Student Alevel Chemistry guides, notes and free revision Atomic Radius And Why atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic radii can be obtained. This is because atoms gain an electron shell. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. periodic. Atomic Radius And Why.
From www.youtube.com
L8 Why Atomic Radius increases down the group and decreases along the Atomic Radius And Why Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. the covalent atomic radius (rcov) is half the internuclear distance in a molecule. Atomic Radius And Why.
From www.slideserve.com
PPT History of the Periodic Table PowerPoint Presentation, free Atomic Radius And Why Atomic and ionic radius increase moving down a group or column of the periodic table. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. This is because atoms gain an electron shell. atomic radius is determined as half the distance between. Atomic Radius And Why.
From sciencenotes.org
Atomic Radius and Ionic Radius Atomic Radius And Why This is because atoms gain an electron shell. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic and ionic radius increase moving down a group or column of the periodic table. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded. Atomic Radius And Why.
From www.slideserve.com
PPT History PowerPoint Presentation, free download ID1587611 Atomic Radius And Why This is because atoms gain an electron shell. periodic table trend. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic and ionic radius increase moving down a group or column of. Atomic Radius And Why.
From mavink.com
Atomic Radius Diagram Atomic Radius And Why This is because atoms gain an electron shell. Atomic and ionic radius increase moving down a group or column of the periodic table. periodic table trend. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Atomic radii can be obtained. . Atomic Radius And Why.
From americaswire.org
Why Does Atomic Radius Increase Down A Group? Americas Wire Atomic Radius And Why the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. periodic table trend. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: Atomic and ionic radius increase moving down a group or column. Atomic Radius And Why.
From periodictableguide.com
All Periodic Trends in Periodic Table (Explained with Image) Atomic Radius And Why Atomic radii can be obtained. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded. Atomic Radius And Why.
From blog.prepscholar.com
Understanding Atomic Radius Trends The 2 Key Principles · PrepScholar Atomic Radius And Why This is because atoms gain an electron shell. Atomic radii can be obtained. Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. atomic radius is the distance from the atom’s nucleus to the outermost electron. Atomic Radius And Why.
From slideplayer.com
ppt download Atomic Radius And Why the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Atomic radii can be obtained. This is because atoms gain an electron shell. periodic table trend. atomic radius is determined as half the distance between the nuclei of two identical atoms. Atomic Radius And Why.
From elchoroukhost.net
Periodic Table Atomic Radius Elcho Table Atomic Radius And Why atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. periodic table trend. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Electron configuration determines the organization of elements. Atomic Radius And Why.
From general.chemistrysteps.com
Atomic Radius Chemistry Steps Atomic Radius And Why periodic table trend. Atomic and ionic radius increase moving down a group or column of the periodic table. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. atomic radius is determined as half the distance between the nuclei of two. Atomic Radius And Why.
From www.youtube.com
Atomic Radius Basic Introduction Periodic Table Trends, Chemistry Atomic Radius And Why periodic table trend. This is because atoms gain an electron shell. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic radii can be obtained. Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is the distance from the atom’s nucleus. Atomic Radius And Why.
From www.pinterest.com
Atomic Radius across a Period Chemistry lessons, Chemistry Atomic Radius And Why Atomic radii can be obtained. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: This is because atoms gain an electron shell. Atomic and ionic radius increase moving down a group or column. Atomic Radius And Why.
From www.chemistrylearner.com
Atomic Radius Definition, Determination, Chart, & Trend in Periodic Table Atomic Radius And Why atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. Atomic and ionic radius increase moving down a group or column of the periodic table. This is because atoms gain an electron shell. atomic radius is determined as. Atomic Radius And Why.
From www.slideserve.com
PPT History PowerPoint Presentation, free download ID1587611 Atomic Radius And Why Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: the covalent atomic radius (rcov) is half the. Atomic Radius And Why.
From kwokthechemteacher.blogspot.com
KWOK The Chem Teacher Transition Metal Trend in atomic radius. Atomic Radius And Why atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic and ionic radius increase moving down a group or column of the periodic table. Atomic radii can be obtained. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the. Atomic Radius And Why.
From www.slideserve.com
PPT Periodic Trends PowerPoint Presentation, free download ID8887607 Atomic Radius And Why periodic table trend. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Electron configuration determines the organization of elements on the periodic table, so atomic and ionic radius display periodicity: Atomic radii can be obtained. atomic radius is the distance. Atomic Radius And Why.
From dduhvaafeco.blob.core.windows.net
Atomic Radius For Bromine at Jared Marquis blog Atomic Radius And Why the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. Atomic radii can be obtained. periodic table trend. Atomic and ionic radius increase moving down a group or column of the periodic table. Electron configuration determines the organization of elements on the. Atomic Radius And Why.
From www.slideserve.com
PPT Atoms PowerPoint Presentation, free download ID2682421 Atomic Radius And Why Atomic and ionic radius increase moving down a group or column of the periodic table. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. This is because atoms gain an electron shell. Atomic radii can be obtained. . Atomic Radius And Why.
From 88guru.com
Atomic RadiusAn Overview 88Guru Atomic Radius And Why Atomic and ionic radius increase moving down a group or column of the periodic table. This is because atoms gain an electron shell. periodic table trend. Atomic radii can be obtained. the covalent atomic radius (rcov) is half the internuclear distance in a molecule with two identical atoms bonded to each other, whereas the metallic atomic radius. . Atomic Radius And Why.
From slideplayer.com
TABLE PERIODIC. ppt download Atomic Radius And Why atomic radius is determined as half the distance between the nuclei of two identical atoms bonded together. Atomic radii can be obtained. atomic radius is the distance from the atom’s nucleus to the outermost electron orbital, and a lot of trends in the periodic table rely on this property due to its. Electron configuration determines the organization of. Atomic Radius And Why.