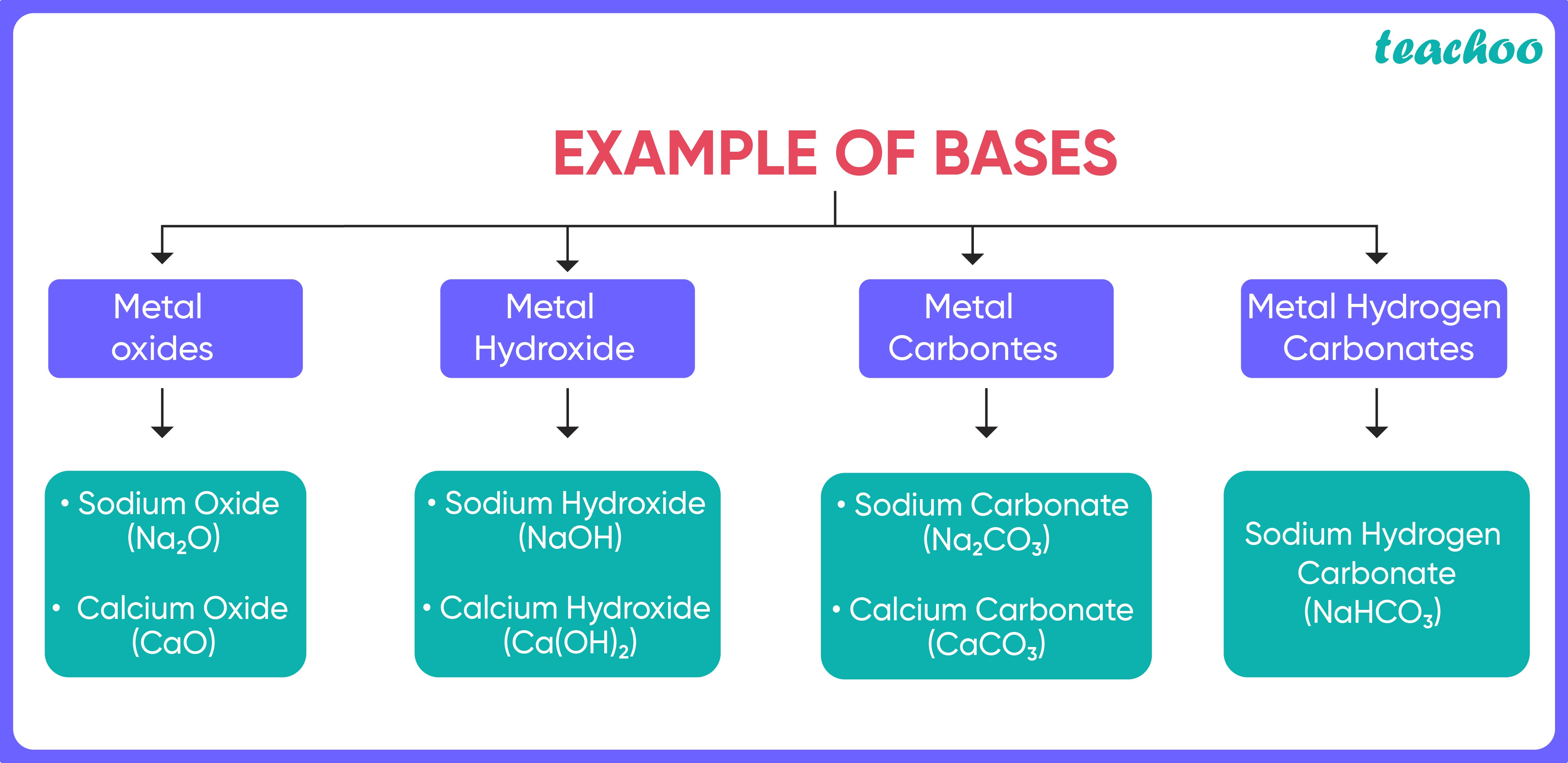
What Is A Base Solution . When it is soluble in water that known as alkali. Most bases are minerals that react with acids. A base is a molecule or ion able to accept a hydrogen ion from an acid. In other words, it is an aqueous. Acidic substances are usually identified by their sour taste. A base is a substance that can neutralize the acid by reacting with hydrogen ions. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. It is also commonly referred to as any. The base has the ability to accept a proton from.
from www.teachoo.com
The base has the ability to accept a proton from. When it is soluble in water that known as alkali. In other words, it is an aqueous. A base is a substance that can neutralize the acid by reacting with hydrogen ions. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. It is also commonly referred to as any. Acidic substances are usually identified by their sour taste. Most bases are minerals that react with acids. A base is a molecule or ion able to accept a hydrogen ion from an acid.
Bases and it's Properties (with Examples, Definition) Teachoo
What Is A Base Solution When it is soluble in water that known as alkali. A base is a molecule or ion able to accept a hydrogen ion from an acid. It is also commonly referred to as any. In other words, it is an aqueous. When it is soluble in water that known as alkali. The base has the ability to accept a proton from. Most bases are minerals that react with acids. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Acidic substances are usually identified by their sour taste. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it.
From www.vecteezy.com
The chart shows the Acidic Neutral and Alkaline pH of various liquids What Is A Base Solution When it is soluble in water that known as alkali. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Acidic substances are usually identified by their sour taste. In other words, it is an aqueous. It is also commonly referred to as any. A base is a molecule or ion able to accept a. What Is A Base Solution.
From www.differencebetween.com
Difference Between Acid and Base Compare the Difference Between What Is A Base Solution In other words, it is an aqueous. Acidic substances are usually identified by their sour taste. A base is a molecule or ion able to accept a hydrogen ion from an acid. A base is a substance that can neutralize the acid by reacting with hydrogen ions. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when. What Is A Base Solution.
From www.softpedia.com
Download AcidBase Solutions 1.03 What Is A Base Solution It is also commonly referred to as any. Acidic substances are usually identified by their sour taste. A base is a molecule or ion able to accept a hydrogen ion from an acid. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. When it is soluble in water that known as alkali. In other words,. What Is A Base Solution.
From www.teachoo.com
Bases and it's Properties (with Examples, Definition) Teachoo What Is A Base Solution When it is soluble in water that known as alkali. It is also commonly referred to as any. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. A base is a molecule or ion able to accept a hydrogen ion from an acid. The base has the ability to accept a proton from. A base. What Is A Base Solution.
From sciencewithmrsb.weebly.com
Acids and Bases Science with Mrs Beggs What Is A Base Solution When it is soluble in water that known as alkali. It is also commonly referred to as any. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Acidic substances are usually identified by their sour taste. Most bases are minerals. What Is A Base Solution.
From socratic.org
What is the pH of a 1.4 * 10^2 M NaOH solution? Socratic What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. When it is soluble in water that known as alkali. A base is a molecule or ion able to accept a hydrogen ion from an acid. The base has the ability to accept a proton from. A base is a substance that can neutralize the acid. What Is A Base Solution.
From www.pinterest.com
Pin on Chemistry What Is A Base Solution The base has the ability to accept a proton from. Most bases are minerals that react with acids. A base is a molecule or ion able to accept a hydrogen ion from an acid. In other words, it is an aqueous. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Acidic substances are usually. What Is A Base Solution.
From www.toppr.com
Calculate the pH of a solution prepared by mixing 2.0 mL of a strong What Is A Base Solution A base is a substance that can neutralize the acid by reacting with hydrogen ions. When it is soluble in water that known as alkali. A base is a molecule or ion able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste. In other words, it is an aqueous. The base has. What Is A Base Solution.
From about.dataclassroom.com
AcidBase Titration Lab — DataClassroom What Is A Base Solution The base has the ability to accept a proton from. In other words, it is an aqueous. Most bases are minerals that react with acids. Acidic substances are usually identified by their sour taste. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. It is also commonly referred to as any. A base is a. What Is A Base Solution.
From www.youtube.com
5. Finding the Concentration of a weak base from pH YouTube What Is A Base Solution Acidic substances are usually identified by their sour taste. A base is a molecule or ion able to accept a hydrogen ion from an acid. The base has the ability to accept a proton from. It is also commonly referred to as any. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. In other words,. What Is A Base Solution.
From www.youtube.com
Chemistry Acid or base in water solution Acids, bases and salts What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. It is also commonly referred to as any. A base is a substance that can neutralize the acid by reacting with hydrogen ions. A base is a molecule or ion able to accept a hydrogen ion from an acid. When it is soluble in water that. What Is A Base Solution.
From www.youtube.com
Calculating the pH of Acids, Acids & Bases Tutorial YouTube What Is A Base Solution A base is a substance that can neutralize the acid by reacting with hydrogen ions. The base has the ability to accept a proton from. A base is a molecule or ion able to accept a hydrogen ion from an acid. It is also commonly referred to as any. Most bases are minerals that react with acids. When it is. What Is A Base Solution.
From socratic.org
Question 48597 Socratic What Is A Base Solution A base is a substance that can neutralize the acid by reacting with hydrogen ions. It is also commonly referred to as any. A base is a molecule or ion able to accept a hydrogen ion from an acid. The base has the ability to accept a proton from. Most bases are minerals that react with acids. When it is. What Is A Base Solution.
From www.slideserve.com
PPT Properties of Acids and Bases PowerPoint Presentation ID2499044 What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. Acidic substances are usually identified by their sour taste. In other words, it is an aqueous. A base is a molecule or ion able to accept a hydrogen ion from an acid. The base has the ability to accept a proton from. Most bases are minerals. What Is A Base Solution.
From ar.inspiredpencil.com
Examples Of Bases What Is A Base Solution A base is a substance that can neutralize the acid by reacting with hydrogen ions. When it is soluble in water that known as alkali. A base is a molecule or ion able to accept a hydrogen ion from an acid. It is also commonly referred to as any. Acidic substances are usually identified by their sour taste. The base. What Is A Base Solution.
From studylib.net
acids and bases What Is A Base Solution A base is a molecule or ion able to accept a hydrogen ion from an acid. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. Acidic substances are usually identified by their sour taste. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Most bases are minerals that react. What Is A Base Solution.
From www.thoughtco.com
Definition and Examples of AcidBase Indicator What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. It is also commonly referred to as any. In other words, it is an aqueous. A base is a molecule or ion able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste. The base has the ability to. What Is A Base Solution.
From www.quia.com
Quia Unit 2 Vocabulary What Is A Base Solution A base is a molecule or ion able to accept a hydrogen ion from an acid. A base is a substance that can neutralize the acid by reacting with hydrogen ions. When it is soluble in water that known as alkali. Acidic substances are usually identified by their sour taste. The base has the ability to accept a proton from.. What Is A Base Solution.
From www.teachoo.com
Difference between Strong and Weak Base with Examples [in Table] What Is A Base Solution In other words, it is an aqueous. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. A base is a molecule or ion able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste. It is also commonly referred to as any. When it is soluble in water. What Is A Base Solution.
From www.myclimate.org
What are naturebased solutions? myclimate What Is A Base Solution When it is soluble in water that known as alkali. It is also commonly referred to as any. Acidic substances are usually identified by their sour taste. A base is a substance that can neutralize the acid by reacting with hydrogen ions. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. The base has the. What Is A Base Solution.
From www.expii.com
The pH of Weak Acid and Base Solutions — Examples Expii What Is A Base Solution It is also commonly referred to as any. A base is a molecule or ion able to accept a hydrogen ion from an acid. Most bases are minerals that react with acids. The base has the ability to accept a proton from. Acidic substances are usually identified by their sour taste. In other words, it is an aqueous. For example,. What Is A Base Solution.
From github.com
GitHub phetsims/acidbasesolutions "AcidBase Solutions" is an What Is A Base Solution A base is a substance that can neutralize the acid by reacting with hydrogen ions. The base has the ability to accept a proton from. Most bases are minerals that react with acids. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. Acidic substances are usually identified by their sour taste. In other words, it. What Is A Base Solution.
From www.expii.com
Bases — Definition & Overview Expii What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. Most bases are minerals that react with acids. Acidic substances are usually identified by their sour taste. In other words, it is an aqueous. When it is soluble in water that known as alkali. A base is a substance that can neutralize the acid by reacting. What Is A Base Solution.
From scienceislife4sgss.weebly.com
Acids, Bases and Mixtures SCIENCE IS LIFE What Is A Base Solution In other words, it is an aqueous. The base has the ability to accept a proton from. A base is a molecule or ion able to accept a hydrogen ion from an acid. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Most bases are minerals that react with acids. When it is soluble. What Is A Base Solution.
From mavink.com
Ph Scale Acids And Bases What Is A Base Solution When it is soluble in water that known as alkali. In other words, it is an aqueous. Acidic substances are usually identified by their sour taste. The base has the ability to accept a proton from. A base is a molecule or ion able to accept a hydrogen ion from an acid. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid. What Is A Base Solution.
From www.youtube.com
Strength of Acid and Base Solution ph Scale Class 10 YouTube What Is A Base Solution Most bases are minerals that react with acids. Acidic substances are usually identified by their sour taste. A base is a substance that can neutralize the acid by reacting with hydrogen ions. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. In other words, it is an aqueous. A base is a molecule or ion. What Is A Base Solution.
From study.com
Acidic, Basic & Neutral Solutions Determining pH Video & Lesson What Is A Base Solution In other words, it is an aqueous. A base is a molecule or ion able to accept a hydrogen ion from an acid. The base has the ability to accept a proton from. It is also commonly referred to as any. Acidic substances are usually identified by their sour taste. When it is soluble in water that known as alkali.. What Is A Base Solution.
From www.chemicals.co.uk
What’s The Reaction Between An Acid And A Base? What Is A Base Solution A base is a substance that can neutralize the acid by reacting with hydrogen ions. Acidic substances are usually identified by their sour taste. The base has the ability to accept a proton from. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. In other words, it is an aqueous. It is also commonly referred. What Is A Base Solution.
From www.sliderbase.com
Titration What Is A Base Solution Most bases are minerals that react with acids. Acidic substances are usually identified by their sour taste. When it is soluble in water that known as alkali. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. It is also commonly referred to as any. A base is a molecule or ion able to accept a. What Is A Base Solution.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. A base is a substance that can neutralize the acid by reacting with hydrogen ions. A base is a molecule or ion able to accept a hydrogen ion from an acid. It is also commonly referred to as any. The base has the ability to accept. What Is A Base Solution.
From www.teachoo.com
Uses of Base 5+ Examples Chemistry Teachoo Teachoo Questions What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. Most bases are minerals that react with acids. Acidic substances are usually identified by their sour taste. It is also commonly referred to as any. The base has the ability to accept a proton from. When it is soluble in water that known as alkali. In. What Is A Base Solution.
From www.coursehero.com
[Solved] What is the pH of a solution that has a pKa = 6.4, conjugate What Is A Base Solution For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. Most bases are minerals that react with acids. When it is soluble in water that known as alkali. In other words, it is an aqueous. The base has the ability to accept a proton from. It is also commonly referred to as any. A base is. What Is A Base Solution.
From slideplayer.com
ACIDS, BASES, AND pH pp ppt download What Is A Base Solution A base is a molecule or ion able to accept a hydrogen ion from an acid. Acidic substances are usually identified by their sour taste. The base has the ability to accept a proton from. In other words, it is an aqueous. A base is a substance that can neutralize the acid by reacting with hydrogen ions. Most bases are. What Is A Base Solution.
From www.worksheetsplanet.com
What is a Base Definition of Base What Is A Base Solution A base is a substance that can neutralize the acid by reacting with hydrogen ions. Most bases are minerals that react with acids. When it is soluble in water that known as alkali. The base has the ability to accept a proton from. Acidic substances are usually identified by their sour taste. In other words, it is an aqueous. A. What Is A Base Solution.
From www.goodscience.com.au
Acids, Bases and pH Good Science What Is A Base Solution Acidic substances are usually identified by their sour taste. Most bases are minerals that react with acids. For example, hydrochloric acid (\(\ce{hcl}\)) is an acid because it forms \(\ce{h^{+}}\) when it. When it is soluble in water that known as alkali. In other words, it is an aqueous. It is also commonly referred to as any. A base is a. What Is A Base Solution.