Why Standard Hydrogen Electrode Potential Is Zero . In theoretical physics, it is the energy of two charges separated by infinity distance. Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The first one is defining a zero for potential. Both conventions also agree on the sign of e for. A standard hydrogen electrode has zero electrode potential because:
from www.chegg.com
The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The first one is defining a zero for potential. In theoretical physics, it is the energy of two charges separated by infinity distance. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Both conventions also agree on the sign of e for. A standard hydrogen electrode has zero electrode potential because: The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v.
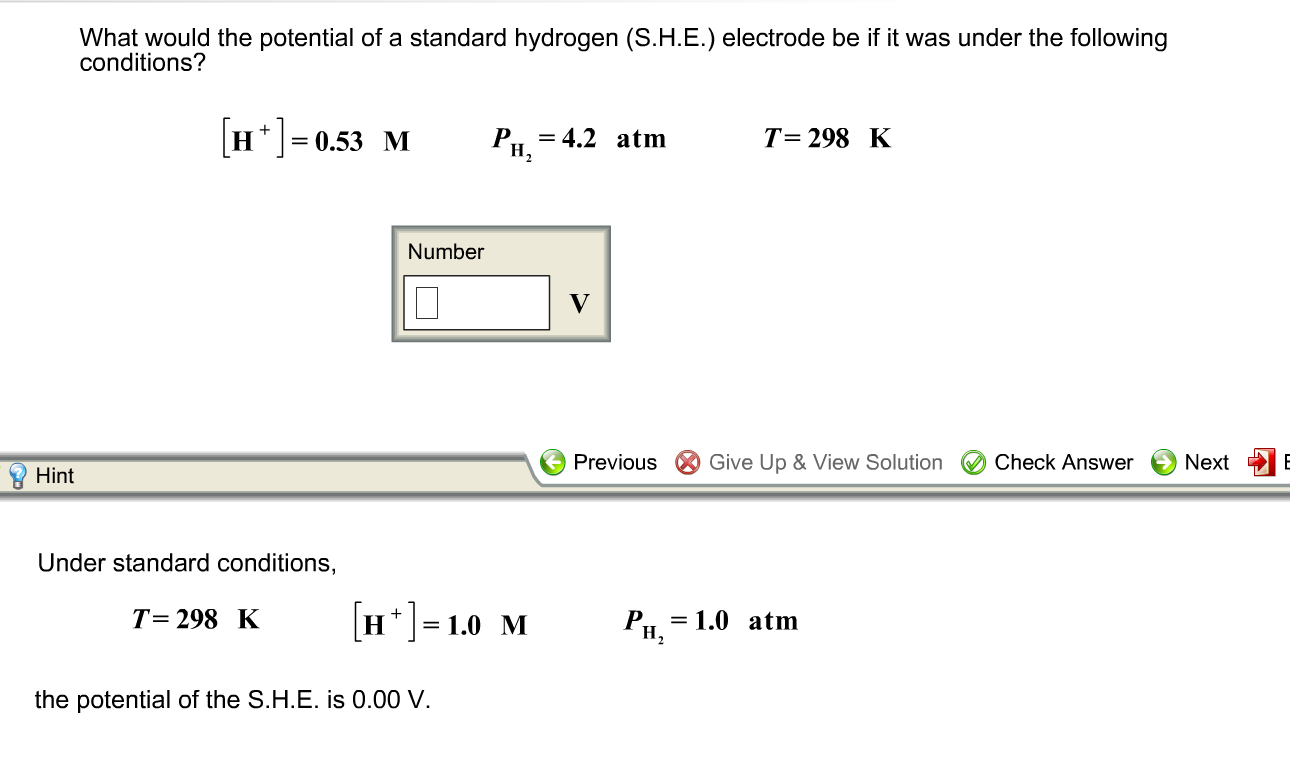
Solved What would the potential of a standard hydrogen
Why Standard Hydrogen Electrode Potential Is Zero The structure of the standard hydrogen electrode is described. A standard hydrogen electrode has zero electrode potential because: The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In theoretical physics, it is the energy of two charges separated by infinity distance. The first one is defining a zero for potential. Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. Both conventions also agree on the sign of e for.
From saylordotorg.github.io
Standard Potentials Why Standard Hydrogen Electrode Potential Is Zero The structure of the standard hydrogen electrode is described. In theoretical physics, it is the energy of two charges separated by infinity distance. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The emf measured when a metal / metal ion electrode is coupled to. Why Standard Hydrogen Electrode Potential Is Zero.
From www.youtube.com
Define standard hydrogen electrode SHE and write the reactions that Why Standard Hydrogen Electrode Potential Is Zero In theoretical physics, it is the energy of two charges separated by infinity distance. The first one is defining a zero for potential. Examples of using the standard hydrogen electrode to determine The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. A standard hydrogen electrode. Why Standard Hydrogen Electrode Potential Is Zero.
From brainly.in
Standard hydrogen electrode explain Brainly.in Why Standard Hydrogen Electrode Potential Is Zero The first one is defining a zero for potential. Both conventions also agree on the sign of e for. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples. Why Standard Hydrogen Electrode Potential Is Zero.
From www.toppr.com
If for an electrode, the standard reduction potential is negative then Why Standard Hydrogen Electrode Potential Is Zero The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The structure of the standard hydrogen electrode is described. Both conventions also agree on the sign of e for. The. Why Standard Hydrogen Electrode Potential Is Zero.
From inspiritvr.com
Calculating Standard Cell Potentials Study Guide Inspirit Why Standard Hydrogen Electrode Potential Is Zero The first one is defining a zero for potential. Examples of using the standard hydrogen electrode to determine The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In theoretical physics, it is the energy of two charges. Why Standard Hydrogen Electrode Potential Is Zero.
From www.slideserve.com
PPT Standard hydrogen electrode PowerPoint Presentation, free Why Standard Hydrogen Electrode Potential Is Zero Both conventions also agree on the sign of e for. Examples of using the standard hydrogen electrode to determine The first one is defining a zero for potential. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In theoretical physics, it is the energy of. Why Standard Hydrogen Electrode Potential Is Zero.
From www.semanticscholar.org
Figure 3 from Standard hydrogen electrode and potential of zero charge Why Standard Hydrogen Electrode Potential Is Zero The first one is defining a zero for potential. Examples of using the standard hydrogen electrode to determine A standard hydrogen electrode has zero electrode potential because: The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In. Why Standard Hydrogen Electrode Potential Is Zero.
From wisc.pb.unizin.org
Day 39 Voltaic Cells, HalfCell Potentials Chemistry 109 Fall 2021 Why Standard Hydrogen Electrode Potential Is Zero The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The first one is defining a zero for potential. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The structure of the standard hydrogen electrode is described. Both conventions. Why Standard Hydrogen Electrode Potential Is Zero.
From www.savemyexams.com
Standard Electrode Potential Edexcel International A Level Chemistry Why Standard Hydrogen Electrode Potential Is Zero The structure of the standard hydrogen electrode is described. A standard hydrogen electrode has zero electrode potential because: Both conventions also agree on the sign of e for. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion.. Why Standard Hydrogen Electrode Potential Is Zero.
From www.youtube.com
Standard Reduction Potentials and the Standard Hydrogen Electrode Why Standard Hydrogen Electrode Potential Is Zero In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In theoretical physics, it is the energy of two charges separated by. Why Standard Hydrogen Electrode Potential Is Zero.
From www.youtube.com
Class 12 Electrochemistry / How electrode potential is measured by Why Standard Hydrogen Electrode Potential Is Zero In theoretical physics, it is the energy of two charges separated by infinity distance. The first one is defining a zero for potential. Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. A standard hydrogen electrode has zero electrode potential because: In both conventions, the standard hydrogen electrode is defined to. Why Standard Hydrogen Electrode Potential Is Zero.
From scienceinfo.com
What is Standard Hydrogen Electrode? Why Standard Hydrogen Electrode Potential Is Zero A standard hydrogen electrode has zero electrode potential because: The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of. Why Standard Hydrogen Electrode Potential Is Zero.
From www.nagwa.com
Question Video Identifying Conditions for Measuring Standard Electrode Why Standard Hydrogen Electrode Potential Is Zero Examples of using the standard hydrogen electrode to determine A standard hydrogen electrode has zero electrode potential because: In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In theoretical. Why Standard Hydrogen Electrode Potential Is Zero.
From www.youtube.com
मानक हाइड्रोजन इलेक्ट्रोड विभव क्या हैStandard hydrogen electrode Why Standard Hydrogen Electrode Potential Is Zero A standard hydrogen electrode has zero electrode potential because: Both conventions also agree on the sign of e for. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The structure of the standard hydrogen electrode is described. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode. Why Standard Hydrogen Electrode Potential Is Zero.
From quizlet.com
ELECTRODE POTENTIAL AND CELL Diagram Quizlet Why Standard Hydrogen Electrode Potential Is Zero In theoretical physics, it is the energy of two charges separated by infinity distance. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In both conventions, the. Why Standard Hydrogen Electrode Potential Is Zero.
From www.youtube.com
MEASUREMENT OF ELECTRODE POTENTIAL USING STANDARD HYDROGEN ELECTRODE Why Standard Hydrogen Electrode Potential Is Zero Examples of using the standard hydrogen electrode to determine The first one is defining a zero for potential. A standard hydrogen electrode has zero electrode potential because: The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Both conventions also agree on the sign of e. Why Standard Hydrogen Electrode Potential Is Zero.
From www.vecteezy.com
A Standard Hydrogen Electrode SHE is an electrode that scientists use Why Standard Hydrogen Electrode Potential Is Zero In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. In theoretical physics, it is the energy of two charges separated by infinity distance. The structure of the standard hydrogen electrode is described. Both conventions also agree on the sign of e for. The emf measured when a metal / metal ion electrode is. Why Standard Hydrogen Electrode Potential Is Zero.
From twitter.com
Digital Kemistry Free Online Chemistry Learning on Twitter "Do you Why Standard Hydrogen Electrode Potential Is Zero A standard hydrogen electrode has zero electrode potential because: In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. The emf measured when a metal / metal ion electrode is. Why Standard Hydrogen Electrode Potential Is Zero.
From gaskatel.de
Usage of the hydrogen electrodes HydroFlex and MiniHydroFlex Gaskatel Why Standard Hydrogen Electrode Potential Is Zero The structure of the standard hydrogen electrode is described. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0. Why Standard Hydrogen Electrode Potential Is Zero.
From www.chegg.com
Solved What would the potential of a standard hydrogen Why Standard Hydrogen Electrode Potential Is Zero Both conventions also agree on the sign of e for. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The emf measured when a metal / metal ion electrode. Why Standard Hydrogen Electrode Potential Is Zero.
From www.chegg.com
Solved what would the potential of a standard hydrogen Why Standard Hydrogen Electrode Potential Is Zero In theoretical physics, it is the energy of two charges separated by infinity distance. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. Examples of using the standard hydrogen electrode to determine In both conventions, the standard. Why Standard Hydrogen Electrode Potential Is Zero.
From brainly.in
what is standard hydrogen electrode draw Brainly.in Why Standard Hydrogen Electrode Potential Is Zero The first one is defining a zero for potential. In theoretical physics, it is the energy of two charges separated by infinity distance. A standard hydrogen electrode has zero electrode potential because: In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The structure of the standard hydrogen electrode is described. Examples of using. Why Standard Hydrogen Electrode Potential Is Zero.
From question.pandai.org
Standard Electrode Potential Why Standard Hydrogen Electrode Potential Is Zero The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The first. Why Standard Hydrogen Electrode Potential Is Zero.
From sulekharanirxiichemistry.blogspot.com
SULEKHA Class XII Chemistry STANDARD HYDROGEN ELECTRODE Why Standard Hydrogen Electrode Potential Is Zero The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. A standard hydrogen electrode has zero electrode potential because: In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The emf measured when a metal / metal ion electrode is. Why Standard Hydrogen Electrode Potential Is Zero.
From www.pinterest.com
Standard Hydrogen Electrode in 2020 Electron configuration, Ideal Why Standard Hydrogen Electrode Potential Is Zero The first one is defining a zero for potential. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The. Why Standard Hydrogen Electrode Potential Is Zero.
From saylordotorg.github.io
Electrochemistry Why Standard Hydrogen Electrode Potential Is Zero The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. In theoretical physics, it is the energy of two charges separated by infinity distance. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential. Why Standard Hydrogen Electrode Potential Is Zero.
From www.slideserve.com
PPT Chapter 20 Electrochemistry PowerPoint Presentation, free Why Standard Hydrogen Electrode Potential Is Zero Both conventions also agree on the sign of e for. A standard hydrogen electrode has zero electrode potential because: The structure of the standard hydrogen electrode is described. In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared. Why Standard Hydrogen Electrode Potential Is Zero.
From www.linstitute.net
CIE A Level Chemistry复习笔记5.3.4 Measuring the Standard Electrode Why Standard Hydrogen Electrode Potential Is Zero A standard hydrogen electrode has zero electrode potential because: The first one is defining a zero for potential. In theoretical physics, it is the energy of two charges separated by infinity distance. The structure of the standard hydrogen electrode is described. The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0. Why Standard Hydrogen Electrode Potential Is Zero.
From www.slideserve.com
PPT Chapter 17 Electrochemistry PowerPoint Presentation, free Why Standard Hydrogen Electrode Potential Is Zero Both conventions also agree on the sign of e for. A standard hydrogen electrode has zero electrode potential because: The first one is defining a zero for potential. Examples of using the standard hydrogen electrode to determine In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The standard hydrogen electrode is often abbreviated. Why Standard Hydrogen Electrode Potential Is Zero.
From pandai.me
Standard Electrode Potential Why Standard Hydrogen Electrode Potential Is Zero A standard hydrogen electrode has zero electrode potential because: In theoretical physics, it is the energy of two charges separated by infinity distance. The structure of the standard hydrogen electrode is described. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that. Why Standard Hydrogen Electrode Potential Is Zero.
From www.slideserve.com
PPT CHEM1612 Pharmacy Week 9 Nernst Equation PowerPoint Why Standard Hydrogen Electrode Potential Is Zero In theoretical physics, it is the energy of two charges separated by infinity distance. The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine In both conventions, the standard hydrogen electrode is defined to have a potential of 0 v. The first one is defining a zero for potential. The emf measured. Why Standard Hydrogen Electrode Potential Is Zero.
From www.thoughtco.com
Standard Hydrogen Electrode Chemistry Definition Why Standard Hydrogen Electrode Potential Is Zero A standard hydrogen electrode has zero electrode potential because: In theoretical physics, it is the energy of two charges separated by infinity distance. The first one is defining a zero for potential. Examples of using the standard hydrogen electrode to determine Both conventions also agree on the sign of e for. The emf measured when a metal / metal ion. Why Standard Hydrogen Electrode Potential Is Zero.
From monomole.com
Standard hydrogen electrode Mono Mole Why Standard Hydrogen Electrode Potential Is Zero Examples of using the standard hydrogen electrode to determine The structure of the standard hydrogen electrode is described. A standard hydrogen electrode has zero electrode potential because: The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The. Why Standard Hydrogen Electrode Potential Is Zero.
From www.youtube.com
Standard Hydrogen Electrode (SHE) Electrode Potential A level Why Standard Hydrogen Electrode Potential Is Zero The structure of the standard hydrogen electrode is described. Examples of using the standard hydrogen electrode to determine The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as the standard electrode potential of that metal / metal ion. The standard hydrogen electrode is often abbreviated to she, and. Why Standard Hydrogen Electrode Potential Is Zero.
From classnotes.org.in
Electrode Potential and E.M.F. of a Galvanic Cell Chemistry, Class 12 Why Standard Hydrogen Electrode Potential Is Zero The standard hydrogen electrode is often abbreviated to she, and its standard electrode potential is declared to be 0 at a temperature of 298k. In theoretical physics, it is the energy of two charges separated by infinity distance. The emf measured when a metal / metal ion electrode is coupled to a hydrogen electrode under standard conditions is known as. Why Standard Hydrogen Electrode Potential Is Zero.