What Happens If Surface Tension Is High . The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Next to mercury, water has the highest surface tension of all commonly occurring liquids. This term is typically used only when the liquid surface is in contact with gas (such as the air). if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet.
from www.worksheetsplanet.com
water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. Next to mercury, water has the highest surface tension of all commonly occurring liquids. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption.
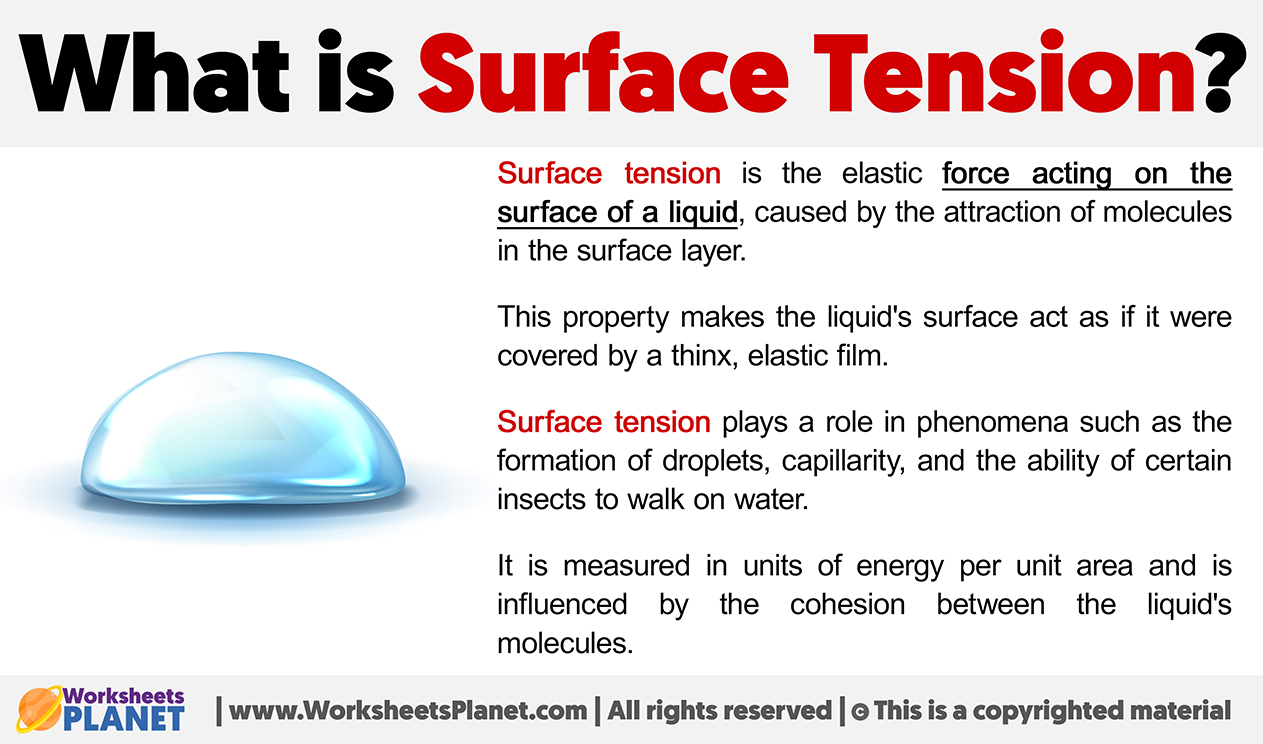
What is Surface Tension?
What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This term is typically used only when the liquid surface is in contact with gas (such as the air). Next to mercury, water has the highest surface tension of all commonly occurring liquids. The cohesive forces between liquid molecules.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Happens If Surface Tension Is High The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. Next to mercury, water has the highest surface tension of all. What Happens If Surface Tension Is High.
From www.slideserve.com
PPT Biomedical importance of surface tension PowerPoint Presentation ID2778612 What Happens If Surface Tension Is High surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with. What Happens If Surface Tension Is High.
From chem.libretexts.org
Surface Tension Chemistry LibreTexts What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components. What Happens If Surface Tension Is High.
From byjus.com
Explain the surface tension phenomenon with examples. What Happens If Surface Tension Is High The cohesive forces between liquid molecules. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has. What Happens If Surface Tension Is High.
From www.geeksforgeeks.org
Surface Tension Definition, Formula, Causes, Examples, and FAQs What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. surface tension is the energy, or work, required to increase the surface. What Happens If Surface Tension Is High.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. The cohesive forces between liquid molecules. Next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in. What Happens If Surface Tension Is High.
From www.biolinscientific.com
Surface tension of water Why is it so high? What Happens If Surface Tension Is High water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. if the surface tension is too high, liquids do not penetrate. What Happens If Surface Tension Is High.
From www.adda247.com
Surface Tension Definition, Formula, Examples What Happens If Surface Tension Is High surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. water, for example, has a very high surface tension,. What Happens If Surface Tension Is High.
From stock.adobe.com
illustration of physics, Surface tension of water, the cohesive forces between water molecules What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is the energy, or work, required to increase the surface area of a liquid due to. What Happens If Surface Tension Is High.
From www.vrogue.co
Surface Tension Of Water Why Is It So High vrogue.co What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a phenomenon in which the surface of a liquid, where the liquid is. What Happens If Surface Tension Is High.
From www.scienceabc.com
Surface Tension Definition, Explanation, Examples And Significance What Happens If Surface Tension Is High surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. This term is typically used only when the liquid surface is in contact with gas (such as the air).. What Happens If Surface Tension Is High.
From www.thoughtco.com
What Is Surface Tension? Definition and Experiments What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. The cohesive forces between liquid molecules. surface tension is a phenomenon in which the surface of a liquid,. What Happens If Surface Tension Is High.
From chemistnotes.com
Surface Tension Definition, Units, Epic Examples, Effects, and Consequences Chemistry Notes What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. The cohesive forces between liquid molecules. surface tension is a phenomenon in which the surface of a liquid, where. What Happens If Surface Tension Is High.
From www.worksheetsplanet.com
What is Surface Tension? What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water. What Happens If Surface Tension Is High.
From www.youtube.com
Surface Tension What is it, how does it form, what properties does it impart YouTube What Happens If Surface Tension Is High water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a. What Happens If Surface Tension Is High.
From learningnadeaumilitia.z21.web.core.windows.net
Does Temperature Affect Surface Tension What Happens If Surface Tension Is High water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. Next to mercury, water has the highest surface tension of all commonly occurring liquids. The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular. What Happens If Surface Tension Is High.
From study.com
Surface Tension Definition, Calculation & Examples Video & Lesson Transcript What Happens If Surface Tension Is High surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is the energy, or work, required to. What Happens If Surface Tension Is High.
From dxorquocc.blob.core.windows.net
What Does High Surface Tension Mean at Freda Bourne blog What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. Next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water, for. What Happens If Surface Tension Is High.
From cartoondealer.com
Surface Tension Explanation Vector Illustration Diagram 175189666 What Happens If Surface Tension Is High water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. The cohesive forces between liquid molecules. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is the energy, or work, required to increase the. What Happens If Surface Tension Is High.
From www.aakash.ac.in
Surface Tension Definition, Causes, Measurement & Formula AESL What Happens If Surface Tension Is High surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. This term is typically used only when the liquid surface is in contact with gas (such as the air). Next to mercury, water has the highest surface tension of all commonly occurring liquids. if the surface tension is. What Happens If Surface Tension Is High.
From www.slideserve.com
PPT Lesson 3 Cohesion, adhesion & surface tension PowerPoint Presentation ID2846820 What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. The cohesive forces between liquid molecules. Next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in. What Happens If Surface Tension Is High.
From www.researchgate.net
a wetting state of surface with low and highsurfacetension fluids b... Download Scientific What Happens If Surface Tension Is High The cohesive forces between liquid molecules. Next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in. What Happens If Surface Tension Is High.
From studiousguy.com
10 Surface Tension Examples in Daily Life StudiousGuy What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). The cohesive forces between liquid molecules. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. if the surface tension is too high, liquids do not penetrate food structures well,. What Happens If Surface Tension Is High.
From dxorquocc.blob.core.windows.net
What Does High Surface Tension Mean at Freda Bourne blog What Happens If Surface Tension Is High The cohesive forces between liquid molecules. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. This term is typically used only when the liquid surface is in contact with gas (such as the air). water, for example, has a very high surface tension, because oxygen and. What Happens If Surface Tension Is High.
From blog.merocourse.com
What is Surface Tension? Factors Affecting Surface Tension Merocourse What Happens If Surface Tension Is High Next to mercury, water has the highest surface tension of all commonly occurring liquids. The cohesive forces between liquid molecules. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. This term is typically used only when the liquid surface is in contact with gas (such as the. What Happens If Surface Tension Is High.
From www.learnatnoon.com
What is Surface Tension in Physics? What Happens If Surface Tension Is High surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. The cohesive forces between liquid molecules. surface tension is the energy,. What Happens If Surface Tension Is High.
From funsizephysics.com
What is Surface Tension? FunsizePhysics What Happens If Surface Tension Is High The cohesive forces between liquid molecules. if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. This term is typically used only when the liquid surface is in contact with gas (such as the air). surface tension is the energy, or work, required to increase the surface. What Happens If Surface Tension Is High.
From byjus.com
On what factor does the magnitude of the surface tension of a liquid depend? What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). The cohesive forces between liquid molecules. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids.. What Happens If Surface Tension Is High.
From www.mdpi.com
Symmetry Free FullText ParticleBased Dynamic Water Drops with High Surface Tension in Real What Happens If Surface Tension Is High Next to mercury, water has the highest surface tension of all commonly occurring liquids. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the. What Happens If Surface Tension Is High.
From www.youtube.com
Surface Tension YouTube What Happens If Surface Tension Is High surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact. What Happens If Surface Tension Is High.
From www.thoughtco.com
Surface Tension Definition in Chemistry What Happens If Surface Tension Is High surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. water, for example, has a very high surface tension, because oxygen and hydrogen—the two chemical components of water (h2o)—have. The cohesive forces between liquid molecules. Next to mercury, water has the highest surface tension of all commonly occurring. What Happens If Surface Tension Is High.
From eduinput.com
Surface TensionDefinition, Examples, Causes, And Measurement What Happens If Surface Tension Is High surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact with a gas, acts as a thin elastic sheet. This term is typically used only when the liquid surface is in contact with gas (such as the air). water, for example, has a very high surface tension, because oxygen and. What Happens If Surface Tension Is High.
From thechemistrynotes.com
Surface Tension Definition, Formula, Unit, Causes, Examples, Consequences What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. Next to mercury, water has the highest surface tension of all commonly occurring liquids. This term is. What Happens If Surface Tension Is High.
From techblog.ctgclean.com
What is Surface Tension? CTG Technical Blog What Happens If Surface Tension Is High if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is the energy, or work, required to increase the surface area of a liquid due to intermolecular forces. surface tension is a phenomenon in which the surface of a liquid, where the liquid is. What Happens If Surface Tension Is High.
From www.sciencefacts.net
Surface Tension Definition, Examples, and Unit What Happens If Surface Tension Is High This term is typically used only when the liquid surface is in contact with gas (such as the air). if the surface tension is too high, liquids do not penetrate food structures well, resulting in less flavor and aroma absorption. surface tension is a phenomenon in which the surface of a liquid, where the liquid is in contact. What Happens If Surface Tension Is High.