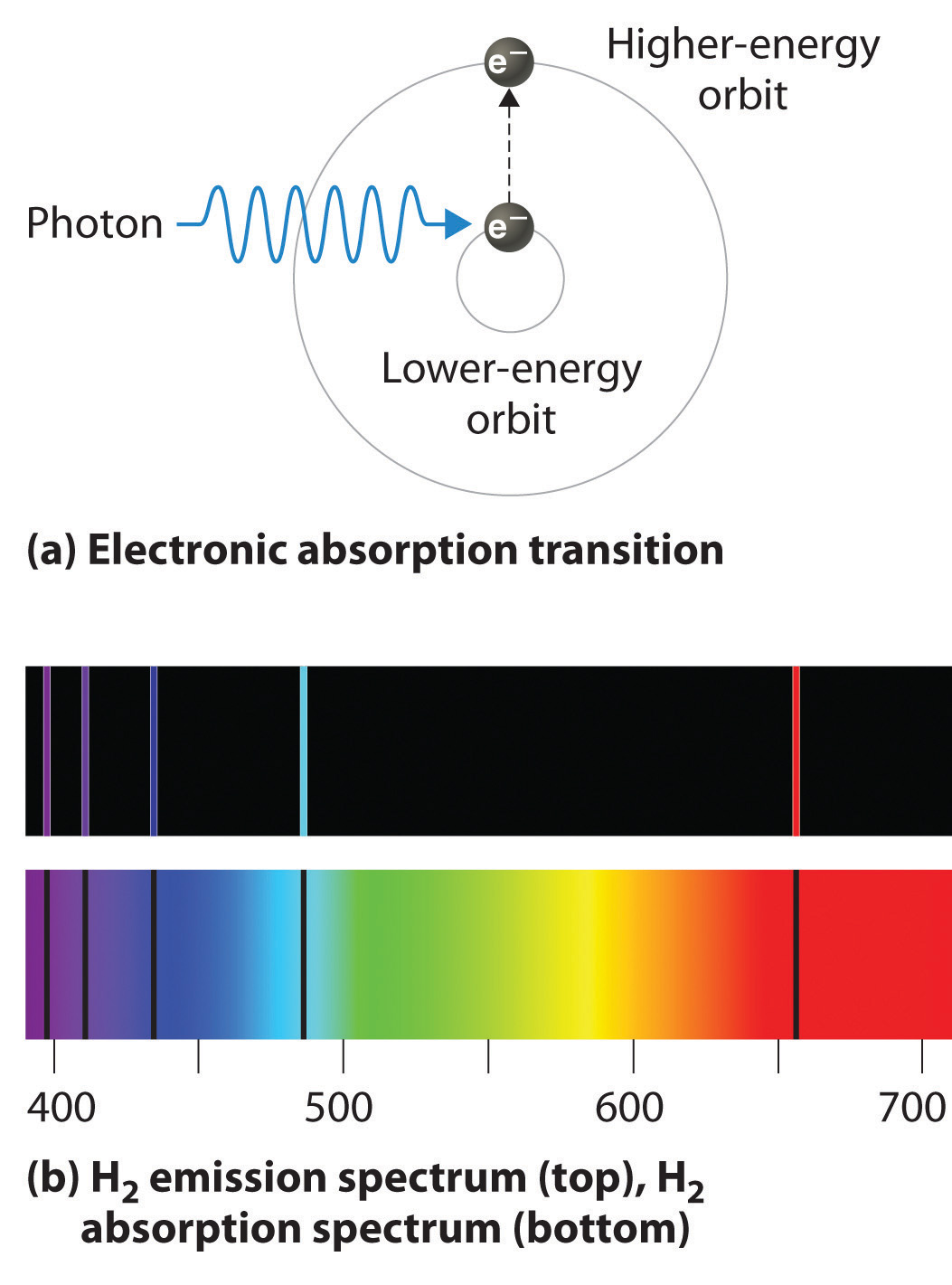
Absorption Spectra Example . Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. When an electron jumps from a. Each element has its own unique absorption spectra, just like it has a unique emission spectra.
from users.highland.edu
Each element has its own unique absorption spectra, just like it has a unique emission spectra. When an electron jumps from a. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels.
Atomic Spectra and Models of the Atom
Absorption Spectra Example We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. When an electron jumps from a. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors.
From mainefilo.weebly.com
Absorption spectra for hydrogen mainefilo Absorption Spectra Example Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed.. Absorption Spectra Example.
From www.researchgate.net
Absorption (orange) and emission (blues) spectra of Coumarin 153(10 Absorption Spectra Example When an electron jumps from a. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. Absorption spectroscopy is a powerful. Absorption Spectra Example.
From www.esa.int
ESA Absorption and emission spectra of various elements Absorption Spectra Example We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Each element has its own unique absorption spectra, just like it has a unique emission. Absorption Spectra Example.
From spiff.rit.edu
Spectrographs and Spectra Absorption Spectra Example We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. This means they can be used to identify. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Each element. Absorption Spectra Example.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. When an electron jumps from a. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Each element has its own unique absorption spectra, just. Absorption Spectra Example.
From hubpages.com
What Is The Difference Between Emission Spectra and Absorption Spectra Absorption Spectra Example Each element has its own unique absorption spectra, just like it has a unique emission spectra. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption. Absorption Spectra Example.
From www.youtube.com
What is Effect of Solvent on UV Absorption Spectra Spectroscopy Absorption Spectra Example Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. This means they can be. Absorption Spectra Example.
From www.vernier.com
A Quantitative Investigation of the Helium Spectrum Absorption Spectra Example Each element has its own unique absorption spectra, just like it has a unique emission spectra. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of. Absorption Spectra Example.
From www.anisotropela.dk
Redshift Absorption Spectra Example When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. This means they can. Absorption Spectra Example.
From www.slideserve.com
PPT Atomic Absorption Spectroscopy PowerPoint Presentation, free Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. When an electron jumps from a. This means they can be used to identify. Each element has its own unique absorption spectra,. Absorption Spectra Example.
From www.researchgate.net
Two typical infrared absorption spectra from each sample showing fine Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic emission spectroscopy measures the. Absorption Spectra Example.
From www.nagwa.com
Lesson Video Emission and Absorption Spectra Nagwa Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This means they can be. Absorption Spectra Example.
From www.slideserve.com
PPT Three Types of Spectra PowerPoint Presentation, free download Absorption Spectra Example When an electron jumps from a. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. This means they can be. Absorption Spectra Example.
From www.edinst.com
What are Absorption, Excitation and Emission Spectra? Absorption Spectra Example Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic emission. Absorption Spectra Example.
From www.slideserve.com
PPT Chapter 42 PowerPoint Presentation, free download ID5580472 Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Each element has its own unique absorption spectra, just like it has a unique emission spectra. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could. Absorption Spectra Example.
From www.mdpi.com
IJMS Free FullText Modulations in Chlorophyll a Fluorescence Based Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. When an electron jumps from a. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic. Absorption Spectra Example.
From www.youtube.com
2.3.2 Distinguish between a continuous spectrum and a line spectrum Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when. Absorption Spectra Example.
From webbtelescope.org
Spectroscopy 101 Types of Spectra and Spectroscopy b Absorption Spectra Example When an electron jumps from a. Each element has its own unique absorption spectra, just like it has a unique emission spectra. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Absorption spectroscopy is a powerful technique to probe fundamental information. Absorption Spectra Example.
From webbtelescope.org
Types of Spectra Continuous, Emission, and Absorption b Absorption Spectra Example Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. When an electron jumps from a. Absorption spectroscopy is a. Absorption Spectra Example.
From www.physicsforums.com
Why are absorption spectra continuous? Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed.. Absorption Spectra Example.
From www.youtube.com
Difference between Action Spectrum and Absorption Spectrum of Absorption Spectra Example Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic. Absorption Spectra Example.
From www.chemistrystudent.com
IR (Infrared Spectroscopy) (ALevel) ChemistryStudent Absorption Spectra Example Each element has its own unique absorption spectra, just like it has a unique emission spectra. This means they can be used to identify. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. When an electron jumps from a. Absorption spectroscopy is a powerful technique to probe fundamental. Absorption Spectra Example.
From www.slideshare.net
Chapter 3 Arrangement of Electrons in the Atom Absorption Spectra Example When an electron jumps from a. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. This means they can be used to identify. Each element has. Absorption Spectra Example.
From www.pinterest.com
The Red Shift of a Hydrogen Absorption Spectrum Wednesday, March 28 Absorption Spectra Example When an electron jumps from a. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic. Absorption Spectra Example.
From www.universetoday.com
Absorption Spectroscopy Universe Today Absorption Spectra Example This means they can be used to identify. Each element has its own unique absorption spectra, just like it has a unique emission spectra. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Atomic emission spectroscopy measures the intensity of light. Absorption Spectra Example.
From www.slideserve.com
PPT Lecture 15 The Hydrogen Atom PowerPoint Presentation ID2710708 Absorption Spectra Example When an electron jumps from a. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic spectra refer to the unique patterns of light. Absorption Spectra Example.
From www.researchgate.net
Normalized absorption and fluorescence spectra of GFP and Cy3. The Absorption Spectra Example We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum of hydrogen in terms of electrons. When an electron jumps from a. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic spectra refer to the unique patterns of light emitted. Absorption Spectra Example.
From www.youtube.com
2.2 Hydrogen emission spectrum (SL) YouTube Absorption Spectra Example When an electron jumps from a. This means they can be used to identify. Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. We developed the bohr model for the hydrogen. Absorption Spectra Example.
From poozacreations.blogspot.com
Types of emission and absorption spectra Pooza Creations Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the electronic energy levels, and could describe the spectrum. Absorption Spectra Example.
From www.researchgate.net
UVVis absorption spectra of the Au NPs Download Scientific Diagram Absorption Spectra Example This means they can be used to identify. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. When an electron jumps from a. Each element has its own unique absorption spectra, just like it has a unique emission spectra. We developed the bohr model for the hydrogen atom. Absorption Spectra Example.
From www.alamy.com
Diagram showing the absorption and emission spectra of the elements Absorption Spectra Example Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. When an electron jumps from a. Each element has its own unique absorption spectra, just like it. Absorption Spectra Example.
From fleminglaser.com.au
Absorption Spectrum (A2) Fleming Laser Absorption Spectra Example Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This means they can be used to identify. Each element has its own unique absorption spectra, just like it has a unique emission spectra. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the. Absorption Spectra Example.
From www.masterorganicchemistry.com
Interpreting IR Specta A Quick Guide Master Organic Chemistry Absorption Spectra Example Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy levels. This means they can be used to identify. We developed the bohr model for the hydrogen atom (section 6.3.3) that quantized the. Absorption Spectra Example.
From users.highland.edu
Atomic Spectra and Models of the Atom Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. When an electron jumps from a. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic spectra refer to the unique patterns of light emitted or absorbed by atoms when their electrons move between different energy. Absorption Spectra Example.
From www.pinterest.com
Absorption spectrum of the elements spectroscopy Physics, Earth and Absorption Spectra Example Absorption spectroscopy is a powerful technique to probe fundamental information on optical transition processes in 2d semiconductors. Each element has its own unique absorption spectra, just like it has a unique emission spectra. Atomic emission spectroscopy measures the intensity of light emitted by the excited atoms, while atomic absorption spectroscopy measures the light absorbed. This means they can be used. Absorption Spectra Example.