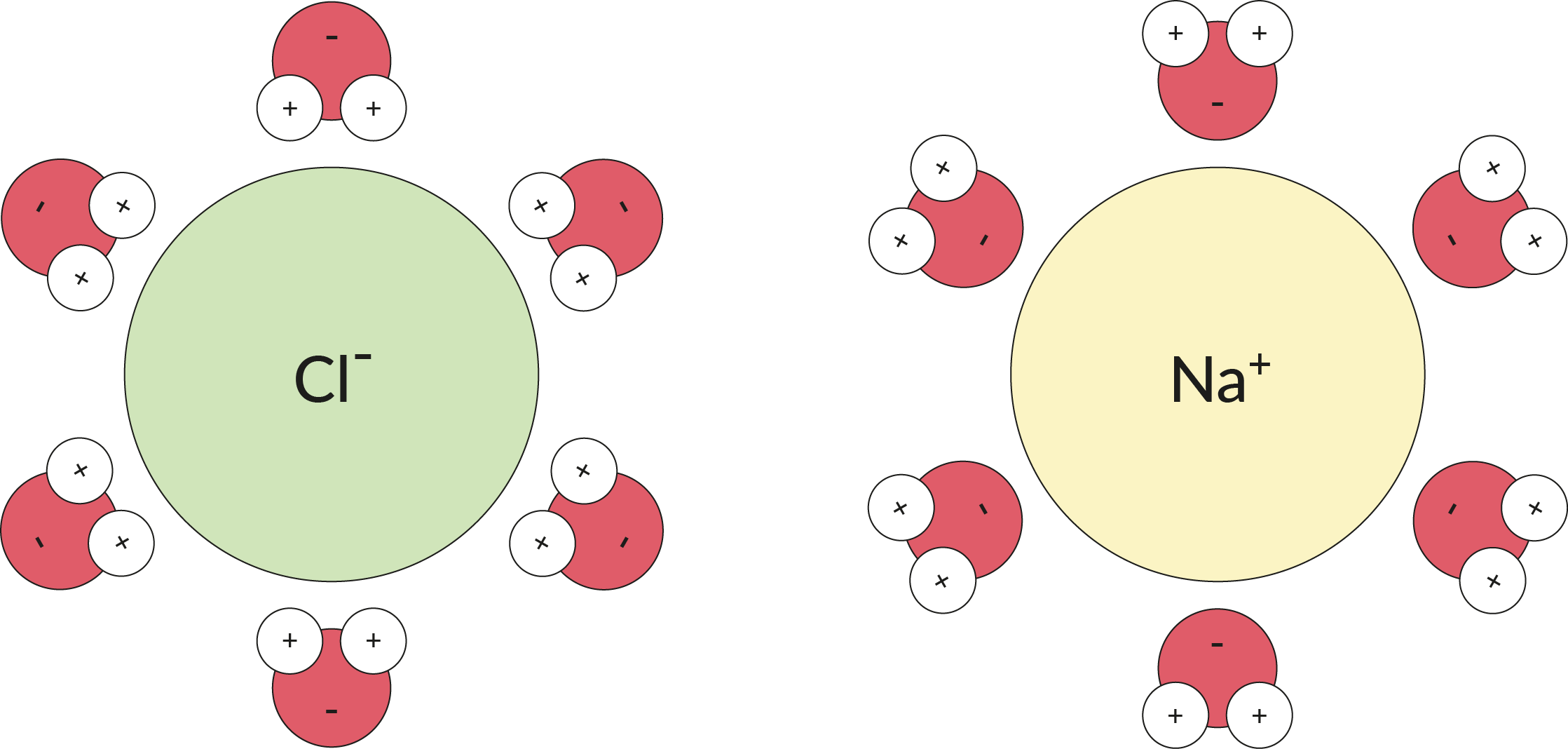
Table Salt Dissolved In Water Mixture . If the salt does not immediately dissolve, try mixing it with a spoon or spatula. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; You need water molecules to come. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. If it is lowered, the water will hold less salt. pour the salt into the water.
from exymmldpm.blob.core.windows.net
If the salt does not immediately dissolve, try mixing it with a spoon or spatula. pour the salt into the water. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. You need water molecules to come. we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. If it is lowered, the water will hold less salt. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity:
Does Salt Dough Dissolve In Water at Charles Barker blog
Table Salt Dissolved In Water Mixture pour the salt into the water. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. pour the salt into the water. If it is lowered, the water will hold less salt. You need water molecules to come. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity:
From www.scienceabc.com
Why Does Sugar Disappear When It Dissolves In Water? » ScienceABC Table Salt Dissolved In Water Mixture You need water molecules to come. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; when table salt is placed in water, the slightly electropositive sodium portion. Table Salt Dissolved In Water Mixture.
From shaunmwilliams.com
Chapter 11 Presentation Table Salt Dissolved In Water Mixture for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; You need water molecules to come. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. pour the salt into the water. chemists use this technique to extract liquids out of a solution, which is. Table Salt Dissolved In Water Mixture.
From www.slideserve.com
PPT BASIC’S OF SOLUTION CHEMISTRY PowerPoint Presentation, free Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound, such as table salt (sodium. If it is lowered, the water will hold less salt. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: when table salt is placed in water, the. Table Salt Dissolved In Water Mixture.
From www.youtube.com
table salt dissolves in water YouTube Table Salt Dissolved In Water Mixture You need water molecules to come. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. pour the salt into the water. we will first examine the process that occurs when an ionic compound, such. Table Salt Dissolved In Water Mixture.
From bio1151.nicerweb.com
solvent.html 03_07DissolvingSaltL.jpg Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound, such as table salt (sodium. You need water molecules to come. we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. when table salt is placed in water, the slightly electropositive sodium portion is. Table Salt Dissolved In Water Mixture.
From www.alamy.com
Salt water in beaker experiment illustration Stock Vector Image & Art Table Salt Dissolved In Water Mixture pour the salt into the water. If it is lowered, the water will hold less salt. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. for table salt, if the temperature is raised, the. Table Salt Dissolved In Water Mixture.
From www.sciencephoto.com
Salt and chalk in water Stock Image C001/0718 Science Photo Library Table Salt Dissolved In Water Mixture pour the salt into the water. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; You need water molecules to come. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. chemists use this technique to extract liquids out of a. Table Salt Dissolved In Water Mixture.
From www.youtube.com
How Salt Dissolves in Water? YouTube Table Salt Dissolved In Water Mixture when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; pour the salt into the water. we will first examine the process that occurs when an ionic compound such as table salt. Table Salt Dissolved In Water Mixture.
From www.wisegeek.com
How Effective Is Salt Water as a Mouthwash? (with pictures) Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound, such as table salt (sodium. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. If the. Table Salt Dissolved In Water Mixture.
From www.science-sparks.com
Fun preschool science Making Mixtures Table Salt Dissolved In Water Mixture If the salt does not immediately dissolve, try mixing it with a spoon or spatula. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. pour the salt. Table Salt Dissolved In Water Mixture.
From exytasiip.blob.core.windows.net
When Table Salt Is Dissolved In Water The Sodium And Chloride Ions at Table Salt Dissolved In Water Mixture If it is lowered, the water will hold less salt. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. chemists use this technique to extract liquids out of a solution, which is. Table Salt Dissolved In Water Mixture.
From exyegvjoc.blob.core.windows.net
Table Salt Dissolved In Water A Homogeneous Or Heterogeneous Mixture at Table Salt Dissolved In Water Mixture pour the salt into the water. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. for table salt, if the temperature is raised, the 100 ml of water can dissolve more. Table Salt Dissolved In Water Mixture.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Table Salt Dissolved In Water Mixture for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. You need water molecules to come. If it is lowered, the water will hold less salt. If the salt does. Table Salt Dissolved In Water Mixture.
From sciencing.com
What Happens When Salt Is Added to Water? Sciencing Table Salt Dissolved In Water Mixture If it is lowered, the water will hold less salt. pour the salt into the water. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. we will first examine the process that occurs when. Table Salt Dissolved In Water Mixture.
From circuitwiringtray.z13.web.core.windows.net
Diagram Of Salt Dissolved In Water Table Salt Dissolved In Water Mixture chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: pour the salt into the water. If it is lowered, the water will hold less salt. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. when table salt is. Table Salt Dissolved In Water Mixture.
From www.youtube.com
table salt dissolves in water YouTube Table Salt Dissolved In Water Mixture pour the salt into the water. If it is lowered, the water will hold less salt. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. for table salt, if the temperature is raised, the. Table Salt Dissolved In Water Mixture.
From www.slideserve.com
PPT Chapter 13 Solutions PowerPoint Presentation, free download ID Table Salt Dissolved In Water Mixture pour the salt into the water. we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: when table salt is placed in water,. Table Salt Dissolved In Water Mixture.
From www.youtube.com
Dissolving Salt in Water YouTube Table Salt Dissolved In Water Mixture pour the salt into the water. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; If the salt does not immediately dissolve, try mixing it with a spoon or spatula. If it is lowered, the water will hold less salt. when table salt is placed in water, the slightly. Table Salt Dissolved In Water Mixture.
From exyegvjoc.blob.core.windows.net
Table Salt Dissolved In Water A Homogeneous Or Heterogeneous Mixture at Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. pour the salt into the water. You need water molecules to come. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: when. Table Salt Dissolved In Water Mixture.
From www.wikidoc.org
Solution wikidoc Table Salt Dissolved In Water Mixture chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: If the salt does not immediately dissolve, try mixing it with a spoon or spatula. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. for table salt,. Table Salt Dissolved In Water Mixture.
From fphoto.photoshelter.com
science chemistry experiment solubility Fundamental Photographs The Table Salt Dissolved In Water Mixture You need water molecules to come. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; If the salt does not immediately dissolve, try mixing it with a spoon or spatula. we will. Table Salt Dissolved In Water Mixture.
From stock.adobe.com
How does sodium chloride (NaCl) dissolve in water Векторный объект Table Salt Dissolved In Water Mixture for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; You need water molecules to come. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. we will. Table Salt Dissolved In Water Mixture.
From www.alamy.com
Solutions. homogeneous mixture. experiment with salt and water Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound, such as table salt (sodium. If it is lowered, the water will hold less salt. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: for table salt, if the temperature is raised,. Table Salt Dissolved In Water Mixture.
From www.thewildwaycoffee.com
Off Kilter // Coffee Blog The Wild Way Coffee Creations Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. pour the salt into the water. we will first examine the process that occurs when an ionic compound, such as table. Table Salt Dissolved In Water Mixture.
From www.slideserve.com
PPT Chapter 4 PowerPoint Presentation, free download ID762021 Table Salt Dissolved In Water Mixture If it is lowered, the water will hold less salt. pour the salt into the water. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; You need water molecules to come. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. If. Table Salt Dissolved In Water Mixture.
From giouxewvc.blob.core.windows.net
Can Water Dissolve Glass at Tracey Gentry blog Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound, such as table salt (sodium. pour the salt into the water. If the salt does not immediately dissolve, try mixing it with a spoon or spatula. we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves. Table Salt Dissolved In Water Mixture.
From brainly.in
The image shows particles of salt dissolved in water. Brainly.in Table Salt Dissolved In Water Mixture If the salt does not immediately dissolve, try mixing it with a spoon or spatula. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; chemists use this technique to extract liquids out. Table Salt Dissolved In Water Mixture.
From byjus.com
Perform an activity to find out how to dissolve a solid in a liquid? Table Salt Dissolved In Water Mixture You need water molecules to come. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; chemists use this technique to extract liquids out of a solution, which is what you are going. Table Salt Dissolved In Water Mixture.
From exymmldpm.blob.core.windows.net
Does Salt Dough Dissolve In Water at Charles Barker blog Table Salt Dissolved In Water Mixture when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. You need water molecules to come. If it is lowered, the water will hold less salt. pour the salt into the water. chemists use this technique to extract liquids out of a solution, which is what you are going to. Table Salt Dissolved In Water Mixture.
From www.koyuncusalt.com
Why Does Salt Dissolve In Water? How to Separate Them Back? Salt Table Salt Dissolved In Water Mixture for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride). Table Salt Dissolved In Water Mixture.
From www.dreamstime.com
Experiment with Salt and Water. Making a Saline Water Solution Stock Table Salt Dissolved In Water Mixture You need water molecules to come. pour the salt into the water. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: we will first examine the. Table Salt Dissolved In Water Mixture.
From schematiclistpact101.z22.web.core.windows.net
Diagram Of Salt Dissolved In Water Table Salt Dissolved In Water Mixture for table salt, if the temperature is raised, the 100 ml of water can dissolve more salt; If it is lowered, the water will hold less salt. pour the salt into the water. we will first examine the process that occurs when an ionic compound, such as table salt (sodium. You need water molecules to come. . Table Salt Dissolved In Water Mixture.
From www.vecteezy.com
Dissolving science experiment with sugar dissolve in water 3188660 Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound, such as table salt (sodium. pour the salt into the water. when table salt is placed in water, the slightly electropositive sodium portion is attracted to the slightly. You need water molecules to come. we will first examine the process that occurs when an. Table Salt Dissolved In Water Mixture.
From gionodtqv.blob.core.windows.net
Can Salt Dissolve In Water To Form A Solution at Alethea Haywood blog Table Salt Dissolved In Water Mixture we will first examine the process that occurs when an ionic compound, such as table salt (sodium. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this activity: You need water molecules to come. when table salt is placed in water, the slightly electropositive sodium portion. Table Salt Dissolved In Water Mixture.
From userdatarheumatics.z21.web.core.windows.net
Chemical Equation For Salt Dissolved In Water Table Salt Dissolved In Water Mixture If it is lowered, the water will hold less salt. You need water molecules to come. we will first examine the process that occurs when an ionic compound such as table salt (sodium chloride) dissolves in water. chemists use this technique to extract liquids out of a solution, which is what you are going to do in this. Table Salt Dissolved In Water Mixture.