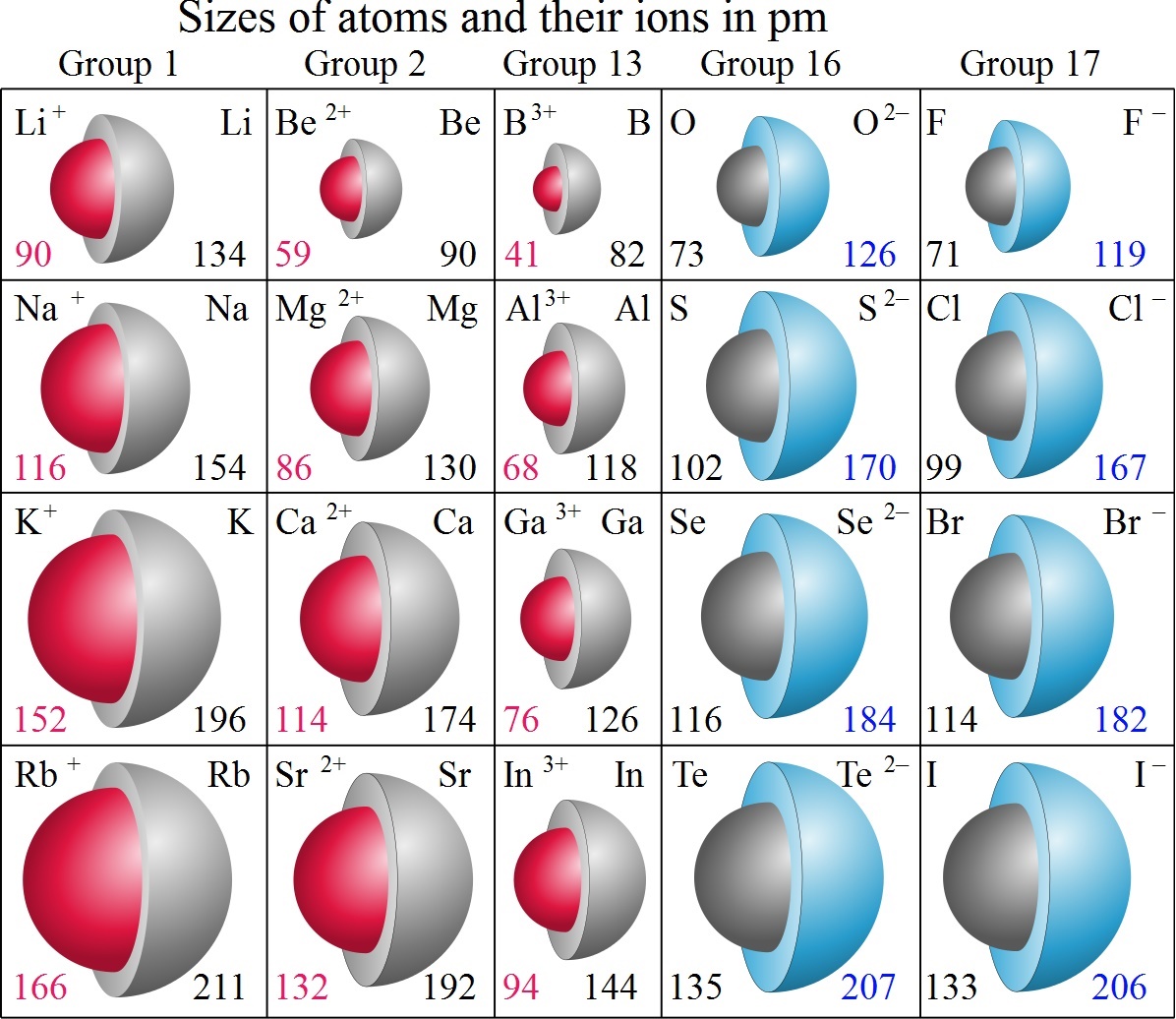
Higher Atomic Radius Chlorine Or Osmium . On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. 119 rows atomic radius of all the elements are mentioned in the chart below. This is caused by electrons being add to higher energy levels that are futher from the nucleus. Atomic radius increases from top to bottom within a group. Compare chlorine vs osmium of the. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Below mentioned radii are the van der waals.
from wwwchem.uwimona.edu.jm
Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Below mentioned radii are the van der waals. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. This is caused by electrons being add to higher energy levels that are futher from the nucleus. Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Compare chlorine vs osmium of the. Atomic radius increases from top to bottom within a group. 119 rows atomic radius of all the elements are mentioned in the chart below. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right).
CHEM1902 Periodic Trends
Higher Atomic Radius Chlorine Or Osmium Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. Compare chlorine vs osmium of the. Atomic radius increases from top to bottom within a group. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Below mentioned radii are the van der waals. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. This is caused by electrons being add to higher energy levels that are futher from the nucleus. 119 rows atomic radius of all the elements are mentioned in the chart below.
From ar.inspiredpencil.com
Measuring Atomic Radius Higher Atomic Radius Chlorine Or Osmium Below mentioned radii are the van der waals. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. Atomic. Higher Atomic Radius Chlorine Or Osmium.
From socratic.org
How does the ionic radius of a typical anion compare? Socratic Higher Atomic Radius Chlorine Or Osmium 119 rows atomic radius of all the elements are mentioned in the chart below. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Below mentioned radii are the van der waals. We assign half of this distance to each chlorine atom, giving chlorine a. Higher Atomic Radius Chlorine Or Osmium.
From hxeqjwlzf.blob.core.windows.net
Size Of Chlorine Atomic Radius at Laura Batts blog Higher Atomic Radius Chlorine Or Osmium This is caused by electrons being add to higher energy levels that are futher from the nucleus. 119 rows atomic radius of all the elements are mentioned in the chart below. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an. Higher Atomic Radius Chlorine Or Osmium.
From www.numerade.com
SOLVED Problem 8 Theoretical Density Calculation (10 points) a Higher Atomic Radius Chlorine Or Osmium On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. 119 rows atomic. Higher Atomic Radius Chlorine Or Osmium.
From material-properties.org
Osmium Periodic Table and Atomic Properties Higher Atomic Radius Chlorine Or Osmium We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. On the periodic table of the elements, atomic radius tends to increase when. Higher Atomic Radius Chlorine Or Osmium.
From www.pathwaystochemistry.com
Atomic and Ionic Radii Pathways to Chemistry Higher Atomic Radius Chlorine Or Osmium Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. For example, sodium in period 3 has an atomic radius of 186 picometers. Higher Atomic Radius Chlorine Or Osmium.
From widiapsari.blogspot.com
Sifatsifat Periodik Unsur Higher Atomic Radius Chlorine Or Osmium Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Below mentioned radii are the van der waals. Atomic radius increases from top to bottom within a group. Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is. Higher Atomic Radius Chlorine Or Osmium.
From classmediacarter.z13.web.core.windows.net
How Do Electronegativity And Polarity Relate Higher Atomic Radius Chlorine Or Osmium Atomic radius increases from top to bottom within a group. This is caused by electrons being add to higher energy levels that are futher from the nucleus. Below mentioned radii are the van der waals. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei. Higher Atomic Radius Chlorine Or Osmium.
From www.sciencephoto.com
Osmium, atomic structure Stock Image C018/3757 Science Photo Library Higher Atomic Radius Chlorine Or Osmium Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Below mentioned radii are the van der waals. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). This is caused by electrons being add to higher energy levels that are futher from the nucleus. Compare chlorine. Higher Atomic Radius Chlorine Or Osmium.
From www.numerade.com
SOLVED Using only the periodic table arrange the following elements in Higher Atomic Radius Chlorine Or Osmium For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. 119 rows atomic radius of all the elements are mentioned in the chart below. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving. Higher Atomic Radius Chlorine Or Osmium.
From www.breakingatom.com
Atomic Radius of Elements Higher Atomic Radius Chlorine Or Osmium Compare chlorine vs osmium of the. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. 119 rows atomic radius of all the elements are mentioned in the chart below. Below mentioned radii are the van der waals. Chlorine has 2 stable naturally occuring. Higher Atomic Radius Chlorine Or Osmium.
From www.chegg.com
Solved Problem If the atomic radius of osmium atoms is 1.34 Higher Atomic Radius Chlorine Or Osmium For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. 119 rows atomic radius of all the elements are mentioned in the chart below. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. We assign half of this distance to each chlorine atom, giving chlorine. Higher Atomic Radius Chlorine Or Osmium.
From socratic.org
How does the atomic radius of argon compare to that of chlorine? Socratic Higher Atomic Radius Chlorine Or Osmium We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. This is caused by electrons being add to higher energy levels that are futher from the nucleus. 119 rows atomic radius of all the elements are mentioned in. Higher Atomic Radius Chlorine Or Osmium.
From www.numerade.com
SOLVED Using only the periodic table arrange the following elements in Higher Atomic Radius Chlorine Or Osmium Compare chlorine vs osmium of the. 119 rows atomic radius of all the elements are mentioned in the chart below. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. On the periodic table of the elements, atomic. Higher Atomic Radius Chlorine Or Osmium.
From www.toppr.com
The first ionisation enthalpy of magnesium is higher than that of Higher Atomic Radius Chlorine Or Osmium Atomic radius increases from top to bottom within a group. This is caused by electrons being add to higher energy levels that are futher from the nucleus. Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which. Higher Atomic Radius Chlorine Or Osmium.
From pediaa.com
Difference Between Atomic Radius and Ionic Radius Higher Atomic Radius Chlorine Or Osmium This is caused by electrons being add to higher energy levels that are futher from the nucleus. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Below mentioned radii are the van der waals. Compare chlorine. Higher Atomic Radius Chlorine Or Osmium.
From www.numerade.com
SOLVED If the atomic radius of osmium atoms is 1.34 × 10−10 m, how Higher Atomic Radius Chlorine Or Osmium Atomic radius increases from top to bottom within a group. 119 rows atomic radius of all the elements are mentioned in the chart below. This is caused by electrons being add to higher energy levels that are futher from the nucleus. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period. Higher Atomic Radius Chlorine Or Osmium.
From ecurrencythailand.com
Which Element Fluorine Or Chlorine Will Have The Greatest Atomic Radius Higher Atomic Radius Chlorine Or Osmium On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has. Higher Atomic Radius Chlorine Or Osmium.
From socratic.org
How does the atomic radius of argon compare to that of chlorine? Socratic Higher Atomic Radius Chlorine Or Osmium Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. Compare chlorine vs osmium of the. Below mentioned radii are the. Higher Atomic Radius Chlorine Or Osmium.
From material-properties.org
Cloro Tabla periódica y propiedades atómicas Higher Atomic Radius Chlorine Or Osmium Compare chlorine vs osmium of the. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 119 rows atomic radius of all the elements are mentioned in the chart below. Below mentioned radii are the van der waals. Atomic radius increases from top to bottom within a group. We assign half of this distance to each chlorine atom, giving chlorine a covalent. Higher Atomic Radius Chlorine Or Osmium.
From www.meritnation.com
Atomic size increases or decreases down a group and from left to right Higher Atomic Radius Chlorine Or Osmium We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. Below mentioned radii are the van der waals. 119 rows atomic radius of all the elements are mentioned in the chart below. On the periodic table of the. Higher Atomic Radius Chlorine Or Osmium.
From www.slideserve.com
PPT Chapter 7 The Structure of Atoms and Periodic Trends PowerPoint Higher Atomic Radius Chlorine Or Osmium Compare chlorine vs osmium of the. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). This is caused by electrons being add to higher energy levels that are futher from the nucleus. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Below mentioned radii are. Higher Atomic Radius Chlorine Or Osmium.
From gia-kharmon.blogspot.com
What Is the Atomic Radius of Chlorine Higher Atomic Radius Chlorine Or Osmium For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Compare chlorine vs osmium of the. Atomic radius increases. Higher Atomic Radius Chlorine Or Osmium.
From brokeasshome.com
Periodic Table Of Elements Sorted By Atomic Radius Higher Atomic Radius Chlorine Or Osmium On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. This is caused. Higher Atomic Radius Chlorine Or Osmium.
From giorzwipc.blob.core.windows.net
Chlorine Sodium Atomic Radius at Nathan McClanahan blog Higher Atomic Radius Chlorine Or Osmium Compare chlorine vs osmium of the. Atomic radius increases from top to bottom within a group. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which. Higher Atomic Radius Chlorine Or Osmium.
From www.sciencephoto.com
Osmium, atomic structure Stock Image C013/1633 Science Photo Library Higher Atomic Radius Chlorine Or Osmium Below mentioned radii are the van der waals. Compare chlorine vs osmium of the. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. This is caused by electrons being. Higher Atomic Radius Chlorine Or Osmium.
From slideplayer.com
Aim What are the Properties of Groups and how does the atomic radius Higher Atomic Radius Chlorine Or Osmium Atomic radius increases from top to bottom within a group. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). This is caused by electrons being add to higher energy levels that are futher from the nucleus. For example, sodium in period 3 has an. Higher Atomic Radius Chlorine Or Osmium.
From hxeqjwlzf.blob.core.windows.net
Size Of Chlorine Atomic Radius at Laura Batts blog Higher Atomic Radius Chlorine Or Osmium This is caused by electrons being add to higher energy levels that are futher from the nucleus. We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. 119 rows atomic radius of all the elements are mentioned in. Higher Atomic Radius Chlorine Or Osmium.
From material-properties.org
Indium Periodic Table and Atomic Properties Higher Atomic Radius Chlorine Or Osmium On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). We assign half of this distance to each chlorine atom, giving chlorine a covalent atomic radius (\(r_{cov}\)), which is half the distance between the nuclei of two like atoms joined by a. This is caused. Higher Atomic Radius Chlorine Or Osmium.
From www.shutterstock.com
585 Chlorine electrons 이미지, 스톡 사진 및 벡터 Shutterstock Higher Atomic Radius Chlorine Or Osmium Below mentioned radii are the van der waals. Atomic radius increases from top to bottom within a group. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Chlorine has 2 stable naturally occuring isotopes while osmium has 6 stable naturally occuring isotopes. This is caused by electrons being add to higher energy levels that are futher from the nucleus. On the. Higher Atomic Radius Chlorine Or Osmium.
From www.nuclear-power.com
Chlorine Atomic Number Atomic Mass Density of Chlorine nuclear Higher Atomic Radius Chlorine Or Osmium Atomic radius increases from top to bottom within a group. 119 rows atomic radius of all the elements are mentioned in the chart below. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. This is caused. Higher Atomic Radius Chlorine Or Osmium.
From www.chegg.com
Solved Place the following elements in order of decreasing Higher Atomic Radius Chlorine Or Osmium 119 rows atomic radius of all the elements are mentioned in the chart below. This is caused by electrons being add to higher energy levels that are futher from the nucleus. Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an. Higher Atomic Radius Chlorine Or Osmium.
From www.quora.com
Which element has an atomic radius smaller than a lithium atom? Quora Higher Atomic Radius Chlorine Or Osmium On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. Below mentioned radii are the van der waals. 119 rows atomic radius of all the elements are mentioned in the chart below. Chlorine has 2 stable naturally. Higher Atomic Radius Chlorine Or Osmium.
From stock.adobe.com
Diagram explaining Atomic Radius using diatomic molecules. Oxygen Higher Atomic Radius Chlorine Or Osmium Below mentioned radii are the van der waals. For example, sodium in period 3 has an atomic radius of 186 picometers and chlorine in the same period has an atomic radius of 99 picometers. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Compare. Higher Atomic Radius Chlorine Or Osmium.
From wwwchem.uwimona.edu.jm
CHEM1902 Periodic Trends Higher Atomic Radius Chlorine Or Osmium This is caused by electrons being add to higher energy levels that are futher from the nucleus. Below mentioned radii are the van der waals. On the periodic table of the elements, atomic radius tends to increase when moving down columns, but decrease when moving across rows (left to right). Sources, facts, uses, scarcity (sri), podcasts, alchemical symbols,. 119 rows. Higher Atomic Radius Chlorine Or Osmium.