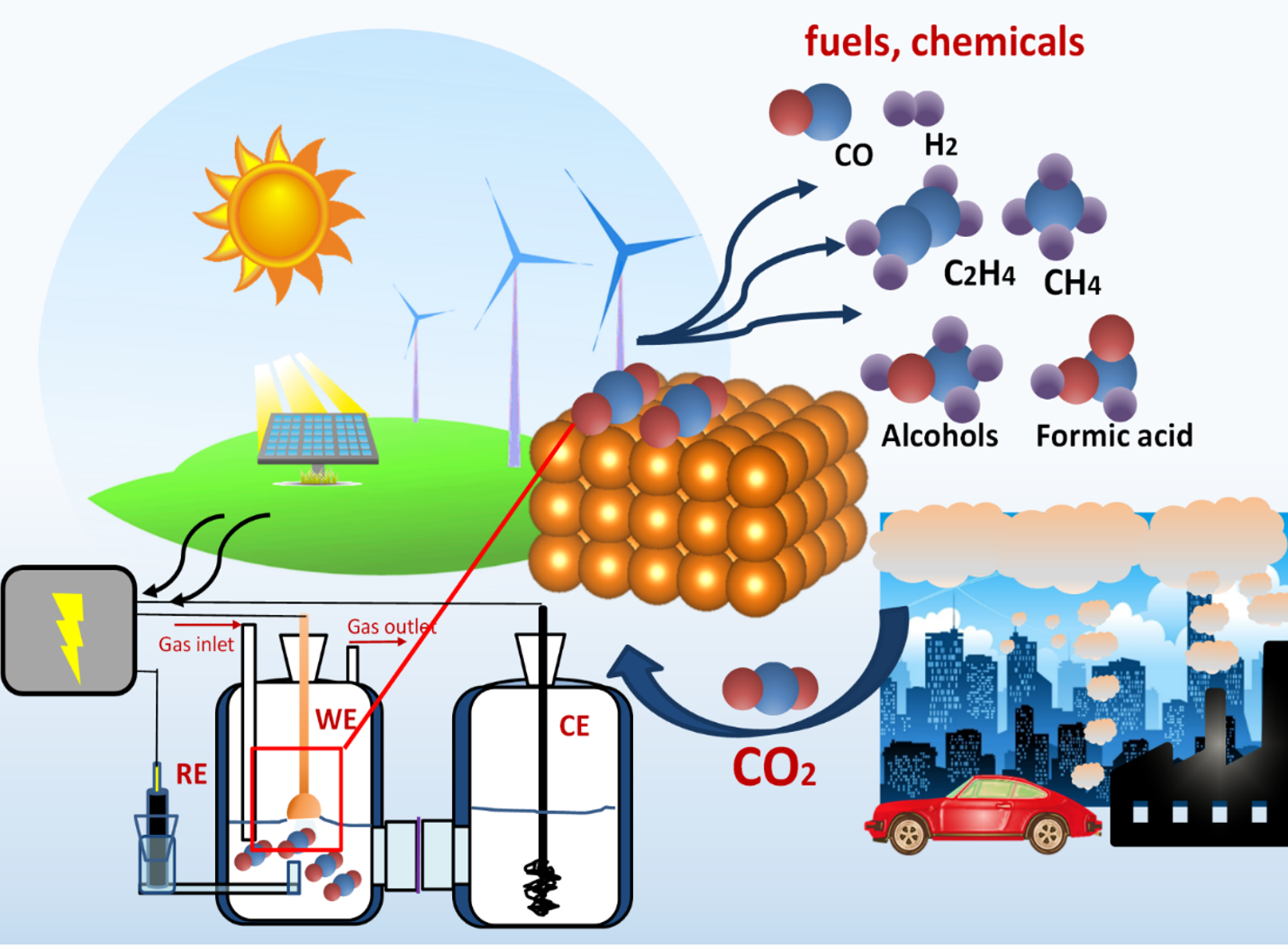
What Is Reduction In Electrochemistry . A reducing agent reduces something else. The oxidized species loses electrons, while the. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. Reduction happens when an atom gains one or more electrons during a chemical reaction. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. This is because an electron has a negative charge,. That means that its oxidation number decreases. Magnesium is good at giving away electrons to. That must mean that it gives electrons to it. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. What is a reduction reaction?
from www.nanoesclab.com
Reduction happens when an atom gains one or more electrons during a chemical reaction. That means that its oxidation number decreases. The oxidized species loses electrons, while the. That must mean that it gives electrons to it. This is because an electron has a negative charge,. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. A reducing agent reduces something else. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. What is a reduction reaction?
Electrochemical reduction of carbon dioxide — NanoESC Lab
What Is Reduction In Electrochemistry The oxidized species loses electrons, while the. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. What is a reduction reaction? That means that its oxidation number decreases. A reducing agent reduces something else. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. Magnesium is good at giving away electrons to. Reduction happens when an atom gains one or more electrons during a chemical reaction. The oxidized species loses electrons, while the. This is because an electron has a negative charge,. That must mean that it gives electrons to it. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as.
From encyclopedia.pub
Fundamentals of the Electroreduction of CO2 Encyclopedia MDPI What Is Reduction In Electrochemistry This is because an electron has a negative charge,. That must mean that it gives electrons to it. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. The oxidized species loses electrons, while the. Magnesium is good at giving away electrons to. This movement of electrons is called electricity, which can. What Is Reduction In Electrochemistry.
From courses.lumenlearning.com
17.3 Standard Reduction Potentials General College Chemistry II What Is Reduction In Electrochemistry This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. That means that its oxidation number decreases. The oxidized species loses electrons, while the. Reduction happens when an atom gains one or more electrons during a chemical reaction. This page discusses the various definitions of. What Is Reduction In Electrochemistry.
From studylib.net
ppt unit 3 Electrochemistry What Is Reduction In Electrochemistry That must mean that it gives electrons to it. A reducing agent reduces something else. Magnesium is good at giving away electrons to. What is a reduction reaction? The oxidized species loses electrons, while the. This is because an electron has a negative charge,. A reduction reaction is one in which a reactant in a chemical reaction gains one or. What Is Reduction In Electrochemistry.
From www.chemicals.co.uk
A Level Chemistry Electrodes & Electrochemical Cells What Is Reduction In Electrochemistry A reducing agent reduces something else. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. Reduction happens when an atom gains one or more electrons during a chemical reaction. That means that its oxidation number decreases. The oxidized species loses electrons, while the. That. What Is Reduction In Electrochemistry.
From pediaa.com
What is the Difference Between Oxidation and Reduction Electrochemical What Is Reduction In Electrochemistry This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. A reducing agent reduces something else. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. This is because an electron has a negative charge,. That means. What Is Reduction In Electrochemistry.
From www.pinterest.co.kr
Standard Potentials in 2023 Electrochemistry, Teaching chemistry What Is Reduction In Electrochemistry This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. This is because an electron has a negative charge,. That must mean that it gives electrons to it. A reducing agent reduces something else. What is a reduction reaction? Magnesium is good at giving away electrons to. The oxidized. What Is Reduction In Electrochemistry.
From www.slideserve.com
PPT Oxidation, Reduction and Electrochemistry PowerPoint Presentation What Is Reduction In Electrochemistry This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. Reduction happens when an atom gains one or more electrons during a chemical reaction. The oxidized species loses electrons, while the. A reducing agent reduces something else. That means that its oxidation number decreases. A. What Is Reduction In Electrochemistry.
From www.youtube.com
19 Electrochemistry Oxidation Reduction Reactions YouTube What Is Reduction In Electrochemistry What is a reduction reaction? The oxidized species loses electrons, while the. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. That must mean that it gives electrons to it. Magnesium is good at giving away electrons to. A reduction reaction is one in. What Is Reduction In Electrochemistry.
From dinorahiu-images.blogspot.com
Standard Potential Table / 18 4 Standard Electrode Potential Powerpoint What Is Reduction In Electrochemistry The oxidized species loses electrons, while the. That must mean that it gives electrons to it. A reducing agent reduces something else. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. That means that its oxidation number decreases. This is because an electron has a negative charge,. This. What Is Reduction In Electrochemistry.
From www.youtube.com
Half cell, oxidation half cell,reduction half cell(Electrochemistry What Is Reduction In Electrochemistry That means that its oxidation number decreases. The oxidized species loses electrons, while the. What is a reduction reaction? Magnesium is good at giving away electrons to. Reduction happens when an atom gains one or more electrons during a chemical reaction. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to. What Is Reduction In Electrochemistry.
From www.researchgate.net
Schematic diagram of the electrochemical CO2 reduction reaction system What Is Reduction In Electrochemistry What is a reduction reaction? That means that its oxidation number decreases. That must mean that it gives electrons to it. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons.. What Is Reduction In Electrochemistry.
From www.vrogue.co
What Is Electrochemical Series vrogue.co What Is Reduction In Electrochemistry A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. What is a reduction reaction? Reduction happens when an atom gains one or more electrons during a chemical reaction. Magnesium is good at giving away electrons to. That must mean that it gives electrons to it. This movement of electrons is called. What Is Reduction In Electrochemistry.
From marco-has-branch.blogspot.com
Which Statement Describes the Reactions in an Electrochemical Cell What Is Reduction In Electrochemistry The oxidized species loses electrons, while the. That must mean that it gives electrons to it. Reduction happens when an atom gains one or more electrons during a chemical reaction. This is because an electron has a negative charge,. What is a reduction reaction? This movement of electrons is called electricity, which can be generated by movements of electrons from. What Is Reduction In Electrochemistry.
From www.nanoesclab.com
Electrochemical reduction of carbon dioxide — NanoESC Lab What Is Reduction In Electrochemistry That must mean that it gives electrons to it. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. A reducing agent reduces something else. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known. What Is Reduction In Electrochemistry.
From www.youtube.com
What is the Difference between Oxidation and Reduction Oxidation What Is Reduction In Electrochemistry That means that its oxidation number decreases. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. What is a reduction reaction? This movement of electrons is called electricity, which can. What Is Reduction In Electrochemistry.
From studylib.net
Oxidation & Reduction Electrochemistry What Is Reduction In Electrochemistry A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. What is a reduction reaction? This movement of electrons is called electricity, which can be generated by movements of electrons from. What Is Reduction In Electrochemistry.
From studylib.net
Electrochemistry What Is Reduction In Electrochemistry The oxidized species loses electrons, while the. Reduction happens when an atom gains one or more electrons during a chemical reaction. Magnesium is good at giving away electrons to. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. A reducing agent reduces something else. That must mean that. What Is Reduction In Electrochemistry.
From socratic.org
Electrolysis "Sn"^(2+) "" "Br"_2 What is the reaction, determine E What Is Reduction In Electrochemistry A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. The oxidized species loses electrons, while the. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. A reducing agent reduces something else. Magnesium is good at giving away electrons to.. What Is Reduction In Electrochemistry.
From wisc.pb.unizin.org
Day 38 OxidationReduction Reactions, Voltaic Cells Chemistry 109 What Is Reduction In Electrochemistry Magnesium is good at giving away electrons to. This is because an electron has a negative charge,. That means that its oxidation number decreases. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. This movement of electrons is called electricity, which can be generated by movements of electrons from one element. What Is Reduction In Electrochemistry.
From www.pinterest.co.uk
Electrochemical series conversation trick to rememberElectrochemistry What Is Reduction In Electrochemistry What is a reduction reaction? That must mean that it gives electrons to it. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. The oxidized species loses electrons, while the. Magnesium is good at giving away electrons to. This movement of electrons is called electricity, which can be. What Is Reduction In Electrochemistry.
From saylordotorg.github.io
Electrochemistry What Is Reduction In Electrochemistry What is a reduction reaction? This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. Magnesium is good at giving away electrons to. Reduction happens when an atom gains one or more electrons during a chemical reaction. This is because an electron has a negative. What Is Reduction In Electrochemistry.
From www.mdpi.com
IJMS Free FullText Highly Selective Electrochemical CO2 Reduction What Is Reduction In Electrochemistry A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. That must mean that it gives electrons to it. What is a reduction reaction? A reducing agent reduces something else. That means that its oxidation number decreases. This movement of electrons is called electricity, which can be generated by movements of electrons. What Is Reduction In Electrochemistry.
From saylordotorg.github.io
Electrochemistry What Is Reduction In Electrochemistry What is a reduction reaction? The oxidized species loses electrons, while the. This is because an electron has a negative charge,. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. A reducing agent reduces something else. A reduction reaction is one in which a reactant in a chemical. What Is Reduction In Electrochemistry.
From www.youtube.com
Oxidation and reduction Electrochemistry Ch3 class 12 What Is Reduction In Electrochemistry The oxidized species loses electrons, while the. A reducing agent reduces something else. That means that its oxidation number decreases. Magnesium is good at giving away electrons to. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. That must mean that it gives electrons to it. This page discusses the various. What Is Reduction In Electrochemistry.
From www.studocu.com
Electrochemistry2 Chimie Electrochemistry Review Oxidation What Is Reduction In Electrochemistry The oxidized species loses electrons, while the. That means that its oxidation number decreases. A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. What is a reduction reaction? This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. A reducing. What Is Reduction In Electrochemistry.
From studylib.net
Introduction to Electrochemistry What Is Reduction In Electrochemistry What is a reduction reaction? This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. Reduction happens when an atom gains one or more electrons during a chemical reaction. This is because an electron has a negative charge,. That means that its oxidation number decreases. This movement of electrons. What Is Reduction In Electrochemistry.
From www.mdpi.com
Electrochem Free FullText Mass Transport Limitations in What Is Reduction In Electrochemistry A reduction reaction is one in which a reactant in a chemical reaction gains one or more electrons. That means that its oxidation number decreases. The oxidized species loses electrons, while the. What is a reduction reaction? This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction. What Is Reduction In Electrochemistry.
From www.mdpi.com
Catalysts Free FullText Recent Advances in Electrochemical What Is Reduction In Electrochemistry This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. The oxidized species loses electrons, while the. What is a reduction reaction? A reduction. What Is Reduction In Electrochemistry.
From www.vrogue.co
Definitions Of Oxidation And Reduction Chemistry Libr vrogue.co What Is Reduction In Electrochemistry Reduction happens when an atom gains one or more electrons during a chemical reaction. The oxidized species loses electrons, while the. That means that its oxidation number decreases. This is because an electron has a negative charge,. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction. What Is Reduction In Electrochemistry.
From www.mdpi.com
Nanomaterials Free FullText ThreeDimensional Cathodes for What Is Reduction In Electrochemistry Reduction happens when an atom gains one or more electrons during a chemical reaction. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. Magnesium is good at giving away electrons to. This movement of electrons is called electricity, which can be generated by movements of electrons from one. What Is Reduction In Electrochemistry.
From www.youtube.com
Standard Reduction Potentials of Half Reactions Electrochemistry What Is Reduction In Electrochemistry A reducing agent reduces something else. That must mean that it gives electrons to it. The oxidized species loses electrons, while the. This is because an electron has a negative charge,. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. That means that its oxidation number decreases. Reduction. What Is Reduction In Electrochemistry.
From mmerevise.co.uk
Calculating the EMF What Is Reduction In Electrochemistry A reducing agent reduces something else. That means that its oxidation number decreases. This page discusses the various definitions of oxidation and reduction (redox) in terms of the transfer of oxygen, hydrogen, and electrons. What is a reduction reaction? This is because an electron has a negative charge,. This movement of electrons is called electricity, which can be generated by. What Is Reduction In Electrochemistry.
From www.dynamicscience.com.au
redox reactions electrolysis The difference between electrochemical What Is Reduction In Electrochemistry This is because an electron has a negative charge,. The oxidized species loses electrons, while the. That means that its oxidation number decreases. That must mean that it gives electrons to it. What is a reduction reaction? A reducing agent reduces something else. This movement of electrons is called electricity, which can be generated by movements of electrons from one. What Is Reduction In Electrochemistry.
From learningcampusstall.z21.web.core.windows.net
Consider The Following Electrochemical Cell What Is Reduction In Electrochemistry A reducing agent reduces something else. Reduction happens when an atom gains one or more electrons during a chemical reaction. This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. The oxidized species loses electrons, while the. This page discusses the various definitions of oxidation. What Is Reduction In Electrochemistry.
From www.youtube.com
Difference between Oxidation and Reduction Redox reaction Class 11 What Is Reduction In Electrochemistry This movement of electrons is called electricity, which can be generated by movements of electrons from one element to another in a reaction known as. Magnesium is good at giving away electrons to. Reduction happens when an atom gains one or more electrons during a chemical reaction. This page discusses the various definitions of oxidation and reduction (redox) in terms. What Is Reduction In Electrochemistry.