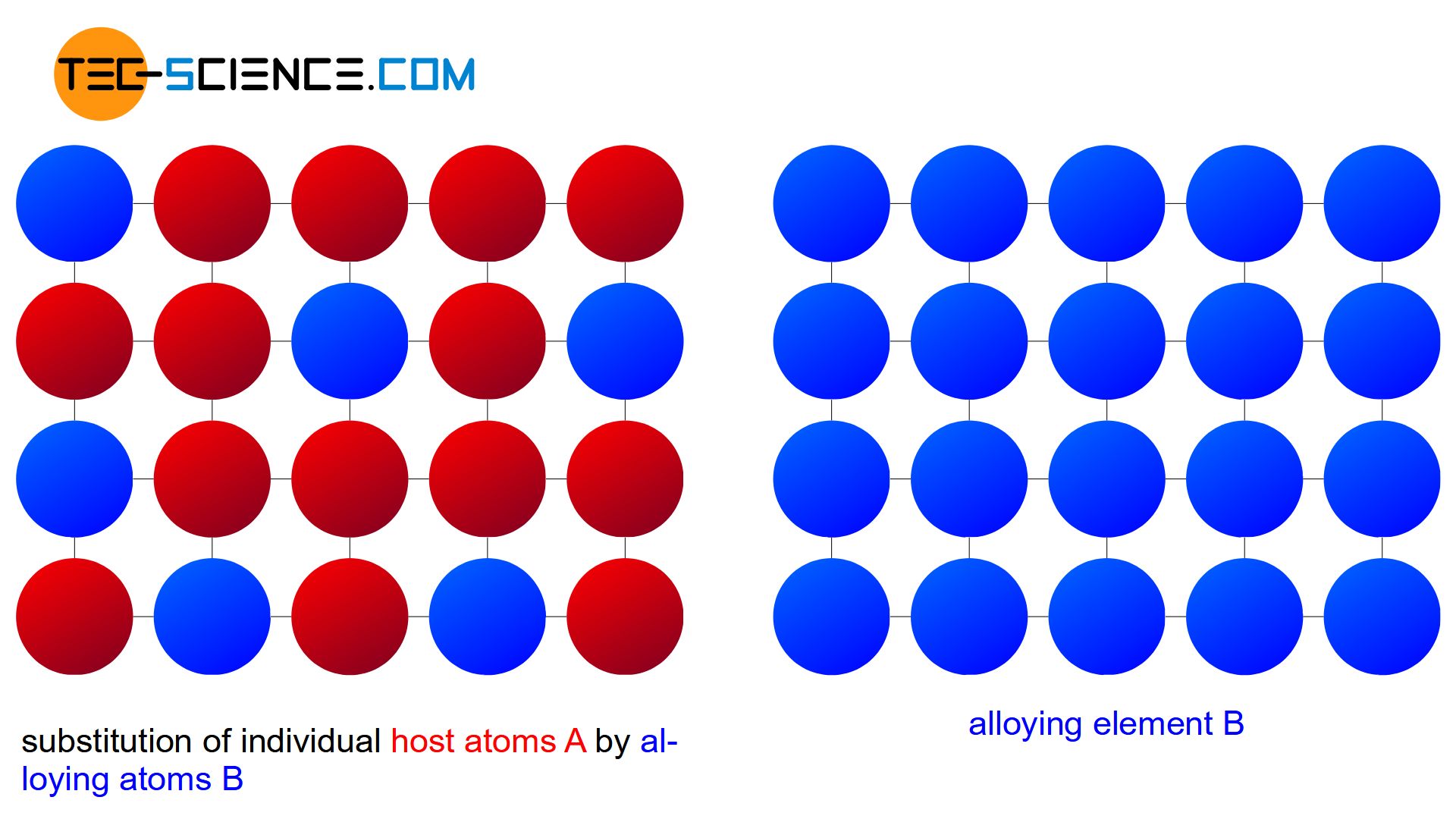
An Alloy Is A Solution In Which . The main component is a metal (often more than one metal) and metallic properties are retained in the new. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. A solid solution can be formed by. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. The components of alloys are ordinarily. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy consists of two or more elements, either as a compound or a solution. The components of alloys are generally metals. An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). An alloy, on the other hand,. An alloy is a mixture of two elemental materials to make a metallic substance. Alloys can be considered as solid.
from www.tec-science.com
An alloy, on the other hand,. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy is a mixture of two elemental materials to make a metallic substance. An alloy consists of two or more elements, either as a compound or a solution. Alloys can be considered as solid. The components of alloys are ordinarily. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. The components of alloys are generally metals. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. The main component is a metal (often more than one metal) and metallic properties are retained in the new.
Typs of alloys tecscience
An Alloy Is A Solution In Which An alloy is a mixture of two elemental materials to make a metallic substance. A solid solution can be formed by. The main component is a metal (often more than one metal) and metallic properties are retained in the new. The components of alloys are ordinarily. An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. Alloys can be considered as solid. An alloy consists of two or more elements, either as a compound or a solution. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. The components of alloys are generally metals. An alloy is a mixture of two elemental materials to make a metallic substance. An alloy, on the other hand,. Alloy, metallic substance composed of two or more elements, as either a compound or a solution.
From www.slideserve.com
PPT Alloys PowerPoint Presentation, free download ID3962254 An Alloy Is A Solution In Which Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. The components of alloys are ordinarily. An alloy consists of two or more elements, either as a compound or a solution. An alloy, on the other hand,. The main component is a metal (often more than one metal) and metallic. An Alloy Is A Solution In Which.
From www.pinterest.com
Intermetallic Compounds vs Solid Solution Alloys Tabular Form Heat energy, Shape memory An Alloy Is A Solution In Which The components of alloys are ordinarily. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. A solid solution can be formed by. The main component is a metal (often more than one metal) and metallic properties are retained in the new. Alloys can be considered as solid. An alloy, on the other hand,.. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT ^_^ MATERIALS ^_^ PowerPoint Presentation, free download ID2255889 An Alloy Is A Solution In Which A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. The components of alloys are ordinarily. An alloy, on the other hand,. Alloys can be considered as solid. The main component is a metal (often more than one metal) and metallic properties are. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Alloys PowerPoint Presentation, free download ID5802468 An Alloy Is A Solution In Which Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. A solid solution can be formed by. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. An alloy, on the other hand,.. An Alloy Is A Solution In Which.
From sciencenotes.org
What Is an Alloy? Definition and Examples An Alloy Is A Solution In Which The components of alloys are generally metals. An alloy, on the other hand,. An alloy consists of two or more elements, either as a compound or a solution. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy is a mixture of two elemental materials to make a metallic substance. A solid. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Crystals PowerPoint Presentation, free download ID152881 An Alloy Is A Solution In Which Alloys can be considered as solid. An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). The components of alloys are ordinarily. The main component is a metal (often more than one metal) and metallic properties are retained in the new. A solid. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation, free download ID1588979 An Alloy Is A Solution In Which An alloy consists of two or more elements, either as a compound or a solution. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms. An Alloy Is A Solution In Which.
From www.tes.com
Alloys Edexcel 91 Separate (Triple) Science Teaching Resources An Alloy Is A Solution In Which The main component is a metal (often more than one metal) and metallic properties are retained in the new. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. An alloy consists of two or more elements, either as a compound or a. An Alloy Is A Solution In Which.
From www.tec-science.com
Typs of alloys tecscience An Alloy Is A Solution In Which An alloy consists of two or more elements, either as a compound or a solution. An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). The components of alloys are ordinarily. The components of alloys are generally metals. The main component is a. An Alloy Is A Solution In Which.
From www.tec-science.com
Typs of alloys tecscience An Alloy Is A Solution In Which An alloy consists of two or more elements, either as a compound or a solution. The main component is a metal (often more than one metal) and metallic properties are retained in the new. An alloy, on the other hand,. The components of alloys are ordinarily. The components of alloys are generally metals. Alloy, metallic substance composed of two or. An Alloy Is A Solution In Which.
From studylib.net
alloysinformation An Alloy Is A Solution In Which A solid solution can be formed by. An alloy is a mixture of two elemental materials to make a metallic substance. An alloy, on the other hand,. An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). The main component is a metal. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Chapter 23 Metals and Metallurgy PowerPoint Presentation, free download ID437433 An Alloy Is A Solution In Which Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. The components of alloys are generally metals. Alloys can be considered as solid. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. A solid solution can be formed by. The main component is. An Alloy Is A Solution In Which.
From msestudent.com
The HumeRothery Rules for Solid Solution Materials Science & Engineering An Alloy Is A Solution In Which An alloy is a mixture of two elemental materials to make a metallic substance. An alloy consists of two or more elements, either as a compound or a solution. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly. An Alloy Is A Solution In Which.
From www.diplomageeks.com
Define alloy and properties of alloy An Alloy Is A Solution In Which An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Chapter 12 Solids and Modern Materials PowerPoint Presentation, free download ID8882980 An Alloy Is A Solution In Which An alloy, on the other hand,. An alloy is a mixture of two elemental materials to make a metallic substance. A solid solution can be formed by. An alloy consists of two or more elements, either as a compound or a solution. The components of alloys are ordinarily. A solid solution refers to a homogeneous mixture of two or more. An Alloy Is A Solution In Which.
From thecontentauthority.com
Solution vs Alloy Differences And Uses For Each One An Alloy Is A Solution In Which An alloy consists of two or more elements, either as a compound or a solution. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. The components of alloys are generally metals. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. A solid. An Alloy Is A Solution In Which.
From www.scribd.com
Alloy & Solid Solution Solution Physical Chemistry An Alloy Is A Solution In Which An alloy is a mixture of two elemental materials to make a metallic substance. An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). An alloy, on the other hand,. Alloys are homogeneous solid solutions in which the atoms of one metal are. An Alloy Is A Solution In Which.
From sciencenotes.org
What Is an Alloy? Definition and Examples An Alloy Is A Solution In Which The components of alloys are generally metals. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. The components of alloys are ordinarily. An alloy, on the other hand,. An alloy consists of two or more elements, either as a compound or a solution. An alloy may be a solid solution of metal elements. An Alloy Is A Solution In Which.
From msestudent.com
The Difference Between Alloys and Composites (and Compounds) Materials Science & Engineering An Alloy Is A Solution In Which Alloys can be considered as solid. An alloy consists of two or more elements, either as a compound or a solution. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level,. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Theory Of alloys PowerPoint Presentation, free download ID5980229 An Alloy Is A Solution In Which The components of alloys are ordinarily. The main component is a metal (often more than one metal) and metallic properties are retained in the new. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. An alloy is a mixture of two elemental materials to make a metallic substance. Alloy,. An Alloy Is A Solution In Which.
From scienceneo.com
Alloys Definition, Types, Properties, Formation, Facts, Uses, and Examples What is an Alloy? An Alloy Is A Solution In Which An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). An alloy is a mixture of two elemental materials to make a metallic substance. The components of alloys are ordinarily. An alloy consists of two or more elements, either as a compound or. An Alloy Is A Solution In Which.
From www.tec-science.com
Typs of alloys tecscience An Alloy Is A Solution In Which A solid solution can be formed by. An alloy is a mixture of two elemental materials to make a metallic substance. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. Alloys are homogeneous solid solutions in which the atoms of one metal. An Alloy Is A Solution In Which.
From www.nagwa.com
Question Video Identifying Why a Solid Alloy Is Described as a Solid Solution Nagwa An Alloy Is A Solution In Which Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy is a mixture of two elemental materials to make a metallic substance. The main component is a metal (often more than one metal) and metallic properties are retained in the new. Alloys can be considered as solid. Alloys are homogeneous solid solutions. An Alloy Is A Solution In Which.
From www.expii.com
Alloys — Overview & Examples Expii An Alloy Is A Solution In Which Alloys can be considered as solid. An alloy, on the other hand,. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. An alloy is a mixture of two elemental materials to make a metallic substance. An alloy consists of two or more. An Alloy Is A Solution In Which.
From www.animalia-life.club
Alloys Chemistry An Alloy Is A Solution In Which An alloy consists of two or more elements, either as a compound or a solution. An alloy is a mixture of two elemental materials to make a metallic substance. A solid solution can be formed by. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys are homogeneous solid solutions in which the. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT SOLUTIONS PowerPoint Presentation, free download ID5979898 An Alloy Is A Solution In Which An alloy, on the other hand,. Alloys are homogeneous solid solutions in which the atoms of one metal are distributed randomly among the atoms of other. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. A solid solution can be formed by.. An Alloy Is A Solution In Which.
From www.tec-science.com
Typs of alloys tecscience An Alloy Is A Solution In Which A solid solution can be formed by. An alloy is a mixture of two elemental materials to make a metallic substance. An alloy consists of two or more elements, either as a compound or a solution. An alloy, on the other hand,. The components of alloys are ordinarily. Alloys can be considered as solid. An alloy may be a solid. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Classifying Matter PowerPoint Presentation, free download ID2765184 An Alloy Is A Solution In Which An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. An alloy, on the other hand,.. An Alloy Is A Solution In Which.
From www.slideserve.com
PPT Metal Casting Processes PowerPoint Presentation, free download ID6659367 An Alloy Is A Solution In Which The main component is a metal (often more than one metal) and metallic properties are retained in the new. An alloy is a mixture of two elemental materials to make a metallic substance. A solid solution can be formed by. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. Alloys are homogeneous solid. An Alloy Is A Solution In Which.
From www.slideshare.net
Alloy An Alloy Is A Solution In Which An alloy may be a solid solution of metal elements (a homogeneous mixture) or a mixture of metallic phases (a heterogeneous mixture of two or more solutions). The components of alloys are ordinarily. An alloy consists of two or more elements, either as a compound or a solution. Alloys can be considered as solid. A solid solution refers to a. An Alloy Is A Solution In Which.
From www.animalia-life.club
Alloys Chemistry An Alloy Is A Solution In Which An alloy consists of two or more elements, either as a compound or a solution. The main component is a metal (often more than one metal) and metallic properties are retained in the new. The components of alloys are ordinarily. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy, on the. An Alloy Is A Solution In Which.
From www.nagwa.com
Question Video Identifying Which Statement Best Describes an Alloy Nagwa An Alloy Is A Solution In Which The components of alloys are ordinarily. The main component is a metal (often more than one metal) and metallic properties are retained in the new. An alloy, on the other hand,. Alloy, metallic substance composed of two or more elements, as either a compound or a solution. An alloy may be a solid solution of metal elements (a homogeneous mixture). An Alloy Is A Solution In Which.
From www.slideserve.com
PPT UNIT I CONSTITUTION OF ALLOYS PowerPoint Presentation, free download ID255823 An Alloy Is A Solution In Which The components of alloys are generally metals. Alloys can be considered as solid. An alloy consists of two or more elements, either as a compound or a solution. An alloy, on the other hand,. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the. An Alloy Is A Solution In Which.
From www.animalia-life.club
Alloys Chemistry An Alloy Is A Solution In Which An alloy is a mixture of two elemental materials to make a metallic substance. Alloys can be considered as solid. A solid solution can be formed by. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. Alloys are homogeneous solid solutions in. An Alloy Is A Solution In Which.
From mavink.com
Solid Solid Solutions Or Alloys An Alloy Is A Solution In Which A solid solution can be formed by. A solid solution refers to a homogeneous mixture of two or more substances at the atomic or molecular level, where one substance is dissolved in the other. The main component is a metal (often more than one metal) and metallic properties are retained in the new. The components of alloys are generally metals.. An Alloy Is A Solution In Which.