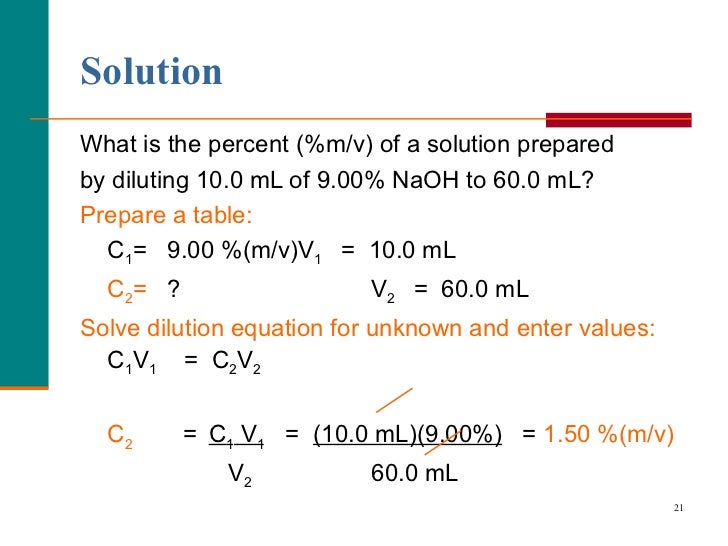
Dilution Formula Percentage . M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). State whether the concentration of a solution is directly or indirectly proportional to its volume. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. You can calculate the concentration of a solution following a dilution by applying this equation:
from www.slideshare.net
Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. The dilution equation allows for the dilution of a stock solution into a working solution. You can calculate the concentration of a solution following a dilution by applying this equation: If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. State whether the concentration of a solution is directly or indirectly proportional to its volume. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer.
Molarity and dilution
Dilution Formula Percentage If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. The dilution equation allows for the dilution of a stock solution into a working solution. You can calculate the concentration of a solution following a dilution by applying this equation: If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). State whether the concentration of a solution is directly or indirectly proportional to its volume.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. You can calculate the concentration of a solution following a dilution. Dilution Formula Percentage.
From www.youtube.com
Dilution, solution, ratio, proportion (laboratory. calculations) YouTube Dilution Formula Percentage If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. The dilution equation allows for the dilution of a stock solution into a. Dilution Formula Percentage.
From www.youtube.com
Dilution calculations Dilution problems Stock dilutions Biology and Dilution Formula Percentage The dilution equation allows for the dilution of a stock solution into a working solution. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. State whether the concentration of a solution is directly or indirectly proportional to. Dilution Formula Percentage.
From slidetodoc.com
Serial Dilution By EID ALATAWI Introduction Many of Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). The dilution equation allows for the dilution of a stock solution into a working solution. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. State whether the concentration of a solution is directly or indirectly. Dilution Formula Percentage.
From www.slideshare.net
Molarity and dilution Dilution Formula Percentage You can calculate the concentration of a solution following a dilution by applying this equation: M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). The dilution equation allows for the dilution of a stock solution. Dilution Formula Percentage.
From materialmagicpayne.z21.web.core.windows.net
How To Calculate Dilution Concentrations Dilution Formula Percentage You can calculate the concentration of a solution following a dilution by applying this equation: Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. M i v i = m f v. Dilution Formula Percentage.
From www.youtube.com
CHEMISTRY 101 Solution Dilutions YouTube Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. State whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula. Dilution Formula Percentage.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). State whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and. Dilution Formula Percentage.
From www.slideserve.com
PPT Chapter 10 Acids and Bases PowerPoint Presentation, free download Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). The dilution equation allows for the dilution of a stock solution into a working solution. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. You can calculate the concentration of a solution following a dilution by applying. Dilution Formula Percentage.
From www.nowfoods.com
Essential Oil Dilution Chart & Calculator NOW Foods Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. You can calculate the concentration of a solution following a dilution by applying this equation: The dilution equation allows for the dilution of a stock solution into. Dilution Formula Percentage.
From www.youtube.com
Dilution Calculation Practice YouTube Dilution Formula Percentage If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. State whether the concentration of a solution is directly or indirectly proportional to its volume. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in. Dilution Formula Percentage.
From www.expii.com
Dilution of Solutions — Overview & Examples Expii Dilution Formula Percentage Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. M i v i = m f v f where m is. Dilution Formula Percentage.
From chem2u.blogspot.com
chem2U Dilution method formula Dilution Formula Percentage The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. You can calculate the concentration of a solution following a dilution by applying this equation: State whether the concentration. Dilution Formula Percentage.
From www.slideshare.net
Molarity and dilution Dilution Formula Percentage You can calculate the concentration of a solution following a dilution by applying this equation: State whether the concentration of a solution is directly or indirectly proportional to its volume. The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration. Dilution Formula Percentage.
From www.youtube.com
DilutionTitration Calculation ChemCram 101 Tutorial YouTube Dilution Formula Percentage If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. The dilution equation allows for the. Dilution Formula Percentage.
From www.youtube.com
Dilution in biology lab How to use the equation C1V1 = C2V2 YouTube Dilution Formula Percentage The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. You can calculate the concentration of a solution following a dilution by applying this equation: If you are starting. Dilution Formula Percentage.
From studylib.net
Dilution Ratios Table Dilution Formula Percentage If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). Calculate appropriate v/v dilution. Dilution Formula Percentage.
From study.com
Dilution Definition, Equation & Factors Video & Lesson Transcript Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. You can calculate the concentration of a solution following a dilution by applying this equation: If you are starting with the solid or liquid material and wish to. Dilution Formula Percentage.
From www.youtube.com
Solution dilution calculations YouTube Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of. Dilution Formula Percentage.
From kayliefernandez.blogspot.com
PreAP Chemistry Dilutions Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. State whether the concentration of a solution is directly or indirectly proportional to its volume. You. Dilution Formula Percentage.
From www.youtube.com
Dilution formula and how to use it to solve problems. 3 examples solved Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). The dilution equation allows for the dilution of a stock solution into a working solution. You can calculate the concentration of a solution following a dilution by applying this equation: If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %,. Dilution Formula Percentage.
From www.youtube.com
Chem 11 Dilution Calculations_Solving for Final Volume after Dilution Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. The dilution equation allows for the dilution of a stock solution into a working solution. If you are starting with the solid or liquid material and. Dilution Formula Percentage.
From www.scientistcindy.com
Dilution Series and Calculations SCIENTIST CINDY Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. State whether the concentration of a solution is directly or indirectly proportional to its volume. You can calculate the concentration of a solution following a dilution by applying this equation: Calculate appropriate v/v dilution using the formula. Dilution Formula Percentage.
From www.slideshare.net
Molarity and dilution Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. State whether the concentration of a solution is directly or indirectly proportional to its volume. You can calculate the concentration of a solution following a dilution by applying this equation: Calculate appropriate v/v dilution using the formula. Dilution Formula Percentage.
From www.slideserve.com
PPT Preparing Solutions with Dilutions PowerPoint Presentation, free Dilution Formula Percentage If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the. Dilution Formula Percentage.
From www.slideserve.com
PPT Chapter 8 Solutions PowerPoint Presentation, free download ID Dilution Formula Percentage The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. You can calculate the concentration of a solution following a dilution by applying this equation: Solution concentration can be. Dilution Formula Percentage.
From www.slideserve.com
PPT Pharmaceutical Calculations (5) PowerPoint Presentation, free Dilution Formula Percentage Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. The dilution equation allows for the dilution of a stock solution into a working solution. If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %,. Dilution Formula Percentage.
From www.slideserve.com
PPT Dilution Calculations PowerPoint Presentation, free download ID Dilution Formula Percentage If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. You can calculate the concentration of a solution following a dilution by applying this equation:. Dilution Formula Percentage.
From www.youtube.com
Dilution and Dilution Factor in Microbiology How to Calculate Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. You can calculate the concentration of a solution following a dilution by applying this equation: Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). If you are starting with the solid or liquid material and. Dilution Formula Percentage.
From www.youtube.com
How to Calculate Dilution Factor YouTube Dilution Formula Percentage State whether the concentration of a solution is directly or indirectly proportional to its volume. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). M i v i = m f v. Dilution Formula Percentage.
From www.youtube.com
Video 18 Simple C1V1 = C2V2 dilution calculations YouTube Dilution Formula Percentage If you wish to perform dilution factor or fold dilution calculations for solutions with molarity or percent concentration units, use our. You can calculate the concentration of a solution following a dilution by applying this equation: The dilution equation allows for the dilution of a stock solution into a working solution. Calculate appropriate v/v dilution using the formula c1v1 =. Dilution Formula Percentage.
From www.educba.com
Dilution Formula Calculator (Examples with Excel Template) Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). If you are starting with the solid or liquid material and wish to make a weight/volume %, weight/weight %, or volume/volume %. You can calculate the concentration of a solution following a dilution by applying this equation: M i v i = m f v f where m is. Dilution Formula Percentage.
From www.youtube.com
Dilution Problems Chemistry Tutorial YouTube Dilution Formula Percentage M i v i = m f v f where m is molarity, v is volume, and the subscripts i and f refer. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. The dilution equation allows for the dilution of a stock solution. Dilution Formula Percentage.
From www.slideserve.com
PPT Concentration of Solutions PowerPoint Presentation, free download Dilution Formula Percentage The dilution equation allows for the dilution of a stock solution into a working solution. Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. State whether the concentration of a solution is. Dilution Formula Percentage.
From www.youtube.com
TRU Chemistry Labs How To do Dilution Calculations YouTube Dilution Formula Percentage Solution concentration can be designated by percentages (%w/w, %w/v and %v/v). State whether the concentration of a solution is directly or indirectly proportional to its volume. Calculate appropriate v/v dilution using the formula c1v1 = c2v2 where c represents the concentration of the solute, and v represents volume in milliliters or ml. If you wish to perform dilution factor or. Dilution Formula Percentage.