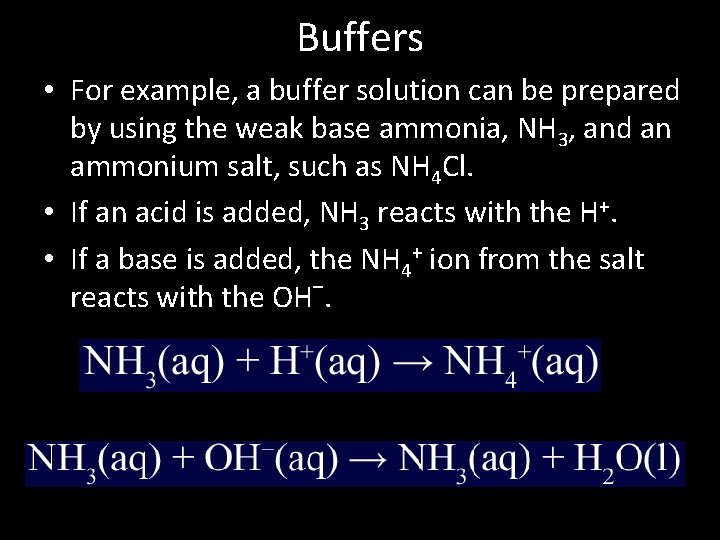
Titration With Buffer Solution . A titration curve is a graph that relates the change in ph of. 1) prepare a buffer solution at a given ph and concentration. 2) analyze the titration curve for the titration of a: Weak acid with a strong. The base (or acid) in the buffer reacts with the added acid (or base). Buffer solutions and titrations are studied in this chapter: A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we.
from slidetodoc.com
A titration curve is a graph that relates the change in ph of. 2) analyze the titration curve for the titration of a: The base (or acid) in the buffer reacts with the added acid (or base). Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. Buffer solutions and titrations are studied in this chapter: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. 1) prepare a buffer solution at a given ph and concentration. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Weak acid with a strong.
Net Ionic Equations Titration Buffers Ch 19 Acids
Titration With Buffer Solution Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A titration curve is a graph that relates the change in ph of. Weak acid with a strong. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Buffer solutions and titrations are studied in this chapter: 2) analyze the titration curve for the titration of a: The base (or acid) in the buffer reacts with the added acid (or base). 1) prepare a buffer solution at a given ph and concentration.
From www.youtube.com
titration with a buffer YouTube Titration With Buffer Solution 2) analyze the titration curve for the titration of a: Buffer solutions and titrations are studied in this chapter: Weak acid with a strong. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and. Titration With Buffer Solution.
From slidetodoc.com
Net Ionic Equations Titration Buffers Ch 19 Acids Titration With Buffer Solution Weak acid with a strong. Buffer solutions and titrations are studied in this chapter: 1) prepare a buffer solution at a given ph and concentration. A titration curve is a graph that relates the change in ph of. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. As seen in the. Titration With Buffer Solution.
From www.sliderbase.com
Buffers Presentation Chemistry Titration With Buffer Solution The base (or acid) in the buffer reacts with the added acid (or base). A titration curve is a graph that relates the change in ph of. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Weak acid with a strong. 2) analyze the titration curve. Titration With Buffer Solution.
From www.youtube.com
Buffers and Titration Curves YouTube Titration With Buffer Solution As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration curve is a graph that relates the change in ph of. 1) prepare a buffer solution at a given ph and concentration. Buffer solutions and titrations are studied in this chapter: Weak acid with a. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Titration curves ph and buffer solutions Studypool Titration With Buffer Solution Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. Buffer solutions and titrations are studied in this chapter: 2) analyze the titration curve for the titration of a: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or.. Titration With Buffer Solution.
From saylordotorg.github.io
Buffers Titration With Buffer Solution Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. 2) analyze the titration curve for the titration of a: Weak acid with a strong. Buffer solutions and titrations are studied in this chapter: 1) prepare a buffer solution at a given ph and concentration. As seen in the chapter on the. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Indicator, Titration of AcidBase and Buffer Solution Study Titration With Buffer Solution 2) analyze the titration curve for the titration of a: Buffer solutions and titrations are studied in this chapter: 1) prepare a buffer solution at a given ph and concentration. A titration curve is a graph that relates the change in ph of. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively. Titration With Buffer Solution.
From slidetodoc.com
AcidBase Titration Buffers Buffers A mixture composed of Titration With Buffer Solution A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. 1) prepare a buffer solution at a given ph and concentration. The base (or acid) in the buffer reacts with the added acid (or base). As seen in the chapter on the stoichiometry of. Titration With Buffer Solution.
From chart-studio.plotly.com
Titration of Phosphate Buffer with Base scatter chart made by Titration With Buffer Solution Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A titration curve is a graph that relates the change in ph of. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. The base (or. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Lecture 10 buffer solutions titrations solubility and common Titration With Buffer Solution A titration curve is a graph that relates the change in ph of. 2) analyze the titration curve for the titration of a: 1) prepare a buffer solution at a given ph and concentration. The base (or acid) in the buffer reacts with the added acid (or base). Weak acid with a strong. As seen in the chapter on the. Titration With Buffer Solution.
From mavink.com
Buffer Region Titration Curve Titration With Buffer Solution A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Weak acid with a strong. A titration curve is a graph that relates the change in ph of. 1) prepare a buffer solution at a given ph and concentration. Now that we have determined. Titration With Buffer Solution.
From www.slideserve.com
PPT Buffers, Titrations, and Aqueous Equilibria PowerPoint Titration With Buffer Solution A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. 2) analyze the titration curve for the titration of a: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A. Titration With Buffer Solution.
From zanenewswaller.blogspot.com
Why Buffer Solution Is Used in Edta Titration Titration With Buffer Solution A titration curve is a graph that relates the change in ph of. Buffer solutions and titrations are studied in this chapter: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Weak acid with a strong. The base (or acid) in the buffer reacts with the. Titration With Buffer Solution.
From favpng.com
Titration Curve Buffer Solution Acidbase Titration PH, PNG, 1024x721px Titration With Buffer Solution Weak acid with a strong. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. 2) analyze the titration curve for the titration of a: Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A buffer solution is. Titration With Buffer Solution.
From www.chemicals.co.uk
Titration Experiments In Chemistry The Chemistry Blog Titration With Buffer Solution Weak acid with a strong. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. The base (or acid) in the buffer reacts with the added acid (or base). A titration curve is a graph that relates the change in ph of. 2) analyze the titration curve for the titration of a:. Titration With Buffer Solution.
From www.scribd.com
Neut Titrations PDF Buffer Solution Ph Titration With Buffer Solution 1) prepare a buffer solution at a given ph and concentration. Weak acid with a strong. A titration curve is a graph that relates the change in ph of. The base (or acid) in the buffer reacts with the added acid (or base). A buffer solution is a solution where the ph does not change significantly on dilution or if. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Indicator, Titration of AcidBase and Buffer Solution Study Titration With Buffer Solution A titration curve is a graph that relates the change in ph of. Weak acid with a strong. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. 1) prepare a buffer solution at a given ph and concentration. Now that we have determined. Titration With Buffer Solution.
From mungfali.com
Buffer Region On Titration Curve Titration With Buffer Solution A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Weak acid with a strong. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A titration curve is a graph that relates the change in. Titration With Buffer Solution.
From www.youtube.com
NCEA L3 Chem Buffers, Buffer Region & Preparation of Buffer Solutions Titration With Buffer Solution Buffer solutions and titrations are studied in this chapter: The base (or acid) in the buffer reacts with the added acid (or base). Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid. Titration With Buffer Solution.
From www.studocu.com
Lecture 5 Exp 4 Buffer Solutions and Titration Curves Lecture 5 Exp Titration With Buffer Solution A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Weak acid with a strong. A titration curve is a graph that relates the change in ph of. Buffer solutions and titrations are studied in this chapter: 1) prepare a buffer solution at a. Titration With Buffer Solution.
From www.scribd.com
lec. 4 buffer solution and titration PDF Chemistry Titration Titration With Buffer Solution 2) analyze the titration curve for the titration of a: Weak acid with a strong. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A titration curve is a graph that relates the change in ph of. Buffer solutions and titrations are studied in this chapter: The base (or acid) in. Titration With Buffer Solution.
From pilgaard.info
Acids and bases Buffers Michael Pilgaard's Chemistry Titration With Buffer Solution Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. Weak acid with a strong. A titration curve is a graph that relates the change in ph of. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at. Titration With Buffer Solution.
From slideplayer.com
Titrations & Buffer solutions ppt download Titration With Buffer Solution A titration curve is a graph that relates the change in ph of. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Buffer solutions and. Titration With Buffer Solution.
From www.scribd.com
Buffer Solution and TITraTION1 PDF Buffer Solution Acid Titration With Buffer Solution 1) prepare a buffer solution at a given ph and concentration. 2) analyze the titration curve for the titration of a: As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Weak acid with a strong. Now that we have determined that there is a mixture of. Titration With Buffer Solution.
From mavink.com
Buffer Region Titration Curve Titration With Buffer Solution A titration curve is a graph that relates the change in ph of. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. The base (or acid) in the buffer reacts with the added acid (or base). 1) prepare a buffer solution at a given ph and. Titration With Buffer Solution.
From pdfslide.net
(PPTX) Buffers and Acid/Base Titration. Buffered Solutions A solution Titration With Buffer Solution The base (or acid) in the buffer reacts with the added acid (or base). A titration curve is a graph that relates the change in ph of. Weak acid with a strong. 2) analyze the titration curve for the titration of a: 1) prepare a buffer solution at a given ph and concentration. Now that we have determined that there. Titration With Buffer Solution.
From ivypanda.com
PH Titrations & Buffer Solutions Experiment 986 Words Essay Example Titration With Buffer Solution The base (or acid) in the buffer reacts with the added acid (or base). 2) analyze the titration curve for the titration of a: Buffer solutions and titrations are studied in this chapter: Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. 1) prepare a buffer solution at a given ph. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Lecture 10 buffer solutions titrations solubility and common Titration With Buffer Solution 2) analyze the titration curve for the titration of a: Weak acid with a strong. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration curve is a graph that relates the change in ph of. A buffer solution is a solution where the ph. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Titration curves ph and buffer solutions Studypool Titration With Buffer Solution Weak acid with a strong. 1) prepare a buffer solution at a given ph and concentration. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature.. Titration With Buffer Solution.
From www.scribd.com
Buffers and Titrations Post PDF Buffer Solution Titration Titration With Buffer Solution The base (or acid) in the buffer reacts with the added acid (or base). A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. As seen. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Titration curves ph and buffer solutions Studypool Titration With Buffer Solution Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. Weak acid with a strong. A titration curve is a graph that relates the change in ph of. The. Titration With Buffer Solution.
From www.slideserve.com
PPT Buffers and Acid/Base Titration PowerPoint Presentation, free Titration With Buffer Solution A titration curve is a graph that relates the change in ph of. Buffer solutions and titrations are studied in this chapter: The base (or acid) in the buffer reacts with the added acid (or base). Weak acid with a strong. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. 1). Titration With Buffer Solution.
From slideplayer.com
Titrations & Buffer solutions ppt download Titration With Buffer Solution Buffer solutions and titrations are studied in this chapter: The base (or acid) in the buffer reacts with the added acid (or base). 1) prepare a buffer solution at a given ph and concentration. Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. A buffer solution is a solution where the. Titration With Buffer Solution.
From www.studypool.com
SOLUTION Titration curves ph and buffer solutions Studypool Titration With Buffer Solution Now that we have determined that there is a mixture of \(\ce{ch_3co_2h}\) and \(\ce{ch3co2^{−}}\) present in solution, we. Weak acid with a strong. As seen in the chapter on the stoichiometry of chemical reactions, titrations can be used to quantitatively analyze solutions for their acid or. A titration curve is a graph that relates the change in ph of. The. Titration With Buffer Solution.
From beta.learner.org
The Buffer System in the Blood (animation) Annenberg Learner Titration With Buffer Solution A titration curve is a graph that relates the change in ph of. A buffer solution is a solution where the ph does not change significantly on dilution or if an acid or base is added at constant temperature. Buffer solutions and titrations are studied in this chapter: The base (or acid) in the buffer reacts with the added acid. Titration With Buffer Solution.