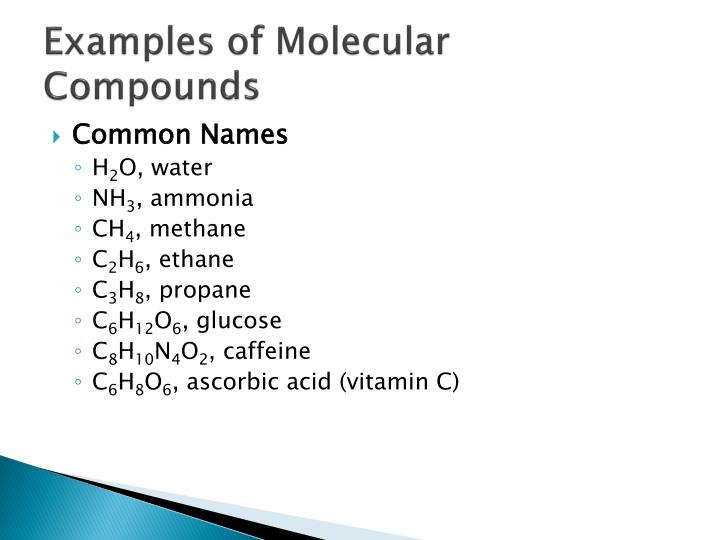
Why Are Molecular Compounds Soft . In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. When an external force or stress is applied to a. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break.
from www.slideserve.com
Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. When an external force or stress is applied to a. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water.
PPT Molecular Compounds PowerPoint Presentation ID2672985
Why Are Molecular Compounds Soft When an external force or stress is applied to a. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. When an external force or stress is applied to a.
From www.slideserve.com
PPT Chemical Nomenclature PowerPoint Presentation, free download ID Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). The shared electrons within the compounds limit electron mobility, so covalent. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Chemical compounds PowerPoint Presentation, free download ID Why Are Molecular Compounds Soft The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. In summary,. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID2938643 Why Are Molecular Compounds Soft When an external force or stress is applied to a. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Chapter 9 Naming Compounds and Writing Formulas PowerPoint Why Are Molecular Compounds Soft An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. When an external force or stress is applied to a. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. The shared electrons within the compounds limit electron mobility, so covalent compounds. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download Why Are Molecular Compounds Soft Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. In summary, covalent compounds are softer, have lower boiling and melting points,. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Chapter 6 Covalent Compounds PowerPoint Presentation, free Why Are Molecular Compounds Soft The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. When an external force or stress is applied to a. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). In summary,. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT 4.2 Representing Molecular Compounds Part I PowerPoint Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). Ionic compounds tend to be hard and brittle while covalent compounds tend to be. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Forming Compounds PowerPoint Presentation, free download ID Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. Classes of molecular solids. Why Are Molecular Compounds Soft.
From www.slideshare.net
Molecular Compounds Why Are Molecular Compounds Soft When an external force or stress is applied to a. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes,. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Chemical Bonding PowerPoint Presentation, free download ID6685692 Why Are Molecular Compounds Soft The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. In summary, covalent compounds are. Why Are Molecular Compounds Soft.
From slidetodoc.com
Molecular Compounds What are molecular compounds and how Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. When an external force or stress is applied to a. The shared electrons within the compounds limit electron mobility, so covalent compounds. Why Are Molecular Compounds Soft.
From sciencenotes.org
What Is a Compound in Chemistry? Definition and Examples Why Are Molecular Compounds Soft An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. When an external force or stress is applied to a. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and. Why Are Molecular Compounds Soft.
From thechemistrynotes.com
Molecular Compounds Important 2 Types, Properties, Uses Why Are Molecular Compounds Soft In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.).. Why Are Molecular Compounds Soft.
From slidetodoc.com
Molecular Compounds and Nomenclature Molecular Nomenclature Most compounds Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. When an external force or stress is applied to a. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Ionic compounds tend to be hard and brittle. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation ID2672985 Why Are Molecular Compounds Soft Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. An ion found in some. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Science 9 Unit B PowerPoint Presentation, free download ID3538308 Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. When an external force or stress is applied to a. Molecular solids are soft, often. Why Are Molecular Compounds Soft.
From ar.inspiredpencil.com
Covalent Solids Why Are Molecular Compounds Soft Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. Ionic compounds tend to be hard and brittle while. Why Are Molecular Compounds Soft.
From www.youtube.com
Lewis Dot Structures for Covalent Compounds Part 1 CLEAR & SIMPLE Why Are Molecular Compounds Soft In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. In. Why Are Molecular Compounds Soft.
From studyingworksheets.com
Molecular Compounds Worksheet Studying Worksheets Why Are Molecular Compounds Soft Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Covalent Bonding PowerPoint Presentation, free download ID2468355 Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens. Why Are Molecular Compounds Soft.
From www.teachoo.com
Molecules and Compounds Definition, Differenences [in Table Form] Why Are Molecular Compounds Soft Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Why Are Molecular Compounds Soft.
From chemistrylabs-2.blogspot.com
Physical Properties Of Molecular Compounds Chemistry Labs Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. Classes of molecular solids. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct. Why Are Molecular Compounds Soft.
From slideplayer.com
Covalent Compounds Molecular Compounds. ppt download Why Are Molecular Compounds Soft Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or. Why Are Molecular Compounds Soft.
From slideplayer.com
Molecular Compounds SNC2D. ppt download Why Are Molecular Compounds Soft The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Solid covalent compounds tend. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Chapter 5 PowerPoint Presentation, free download ID1754973 Why Are Molecular Compounds Soft In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. The shared electrons within the compounds limit electron mobility,. Why Are Molecular Compounds Soft.
From slideplayer.com
Chapter 9 Covalent Bonding ppt download Why Are Molecular Compounds Soft Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. The. Why Are Molecular Compounds Soft.
From slidetodoc.com
Chapter 8 Molecular Compounds Molecular Compounds Covalent bondsAtoms Why Are Molecular Compounds Soft In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. In contrast to ionic compounds, molecular compounds that are solid are soft and flexible. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Molecular solids are soft,. Why Are Molecular Compounds Soft.
From www.slideshare.net
Molecular Compounds Why Are Molecular Compounds Soft Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Compounds PowerPoint Presentation, free download ID2435094 Why Are Molecular Compounds Soft Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water. Molecular solids are soft, often volatile, have low melting temperatures, and are electrical insulators. When an external force or stress is applied. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Molecular Compounds PowerPoint Presentation, free download ID Why Are Molecular Compounds Soft An ion found in some compounds used as antiperspirants contains 13 protons and 10 electrons. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. In contrast to ionic. Why Are Molecular Compounds Soft.
From slideplayer.com
Molecular Compounds and their Covalent Bonds ppt download Why Are Molecular Compounds Soft Solid covalent compounds tend to be soft or brittle because the covalent bonds (and sometimes hydrogen bonds) are relatively easy to break. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). In contrast to ionic compounds, molecular compounds that are solid are soft. Why Are Molecular Compounds Soft.
From www.slideserve.com
PPT Naming Chemical Compounds PowerPoint Presentation, free download Why Are Molecular Compounds Soft When an external force or stress is applied to a. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f, cl, etc.), chalcogens (o, s, etc.), and pnictogens (n, p, etc.). Ionic. Why Are Molecular Compounds Soft.
From www.youtube.com
How to Name Molecular Compounds Examples and Practice Problems YouTube Why Are Molecular Compounds Soft When an external force or stress is applied to a. Ionic compounds tend to be hard and brittle while covalent compounds tend to be softer and more flexible. The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. Molecular solids are soft, often volatile, have low melting temperatures,. Why Are Molecular Compounds Soft.
From slideplayer.com
Covalent Bonds Chapter 5 Section ppt download Why Are Molecular Compounds Soft The shared electrons within the compounds limit electron mobility, so covalent compounds don’t conduct heat or electricity as well as ionic compounds. In summary, covalent compounds are softer, have lower boiling and melting points, are more flammable, are less soluble in water and do not. Classes of molecular solids include organic compounds composed of carbon and hydrogen, fullerenes, halogens (f,. Why Are Molecular Compounds Soft.