Mixed Gas Formula . One of the properties of gases is that they mix with each other. When they do so, they become a solution—a homogeneous mixture. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). \ (p_0\) is not to be confused with the constant \ (p^o\).) By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. (we denote the common initial pressure by “\ (p_0\)”. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. Mixture of gases are common in many.
from www.youtube.com
When they do so, they become a solution—a homogeneous mixture. By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. Mixture of gases are common in many. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). \ (p_0\) is not to be confused with the constant \ (p^o\).) According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. (we denote the common initial pressure by “\ (p_0\)”. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. One of the properties of gases is that they mix with each other.
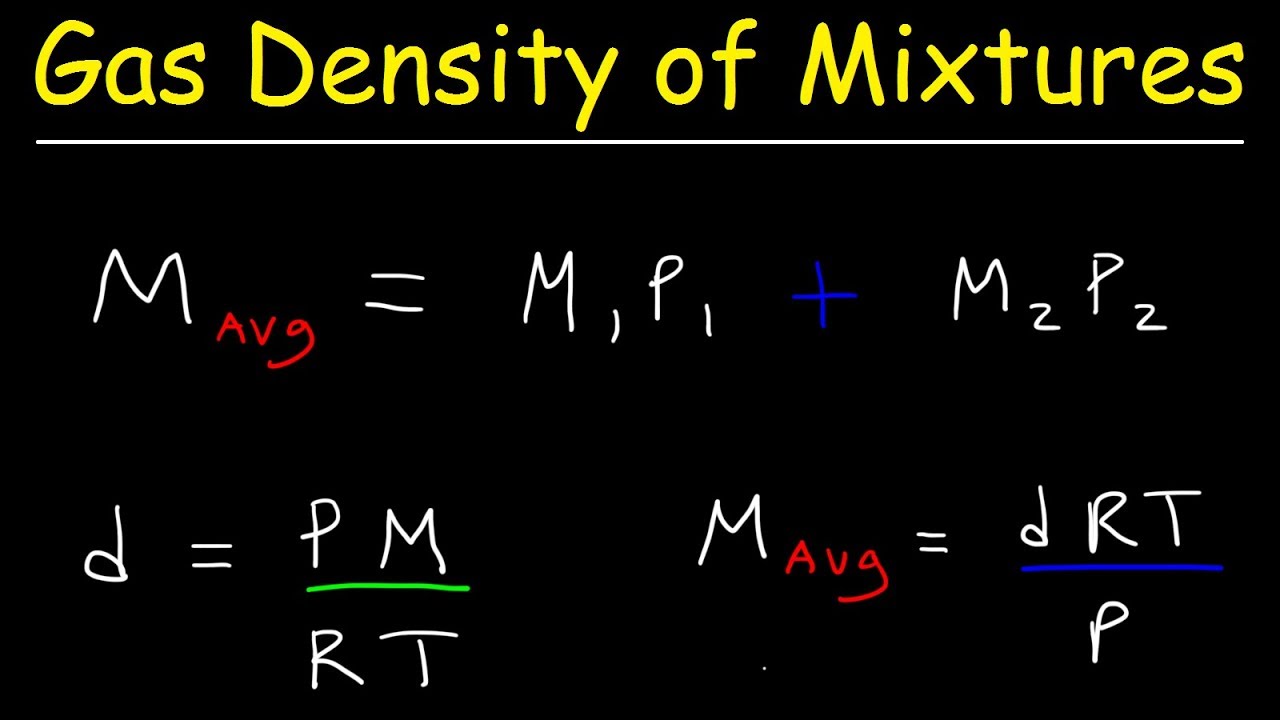
Gas Density & Average Molar Mass of a Gaseous Mixture, Mole Fraction
Mixed Gas Formula According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. (we denote the common initial pressure by “\ (p_0\)”. \ (p_0\) is not to be confused with the constant \ (p^o\).) When they do so, they become a solution—a homogeneous mixture. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. Mixture of gases are common in many. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. One of the properties of gases is that they mix with each other.
From studylib.net
MIXED GAS LAWS WORKSHEET Mixed Gas Formula (we denote the common initial pressure by “\ (p_0\)”. Mixture of gases are common in many. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in. Mixed Gas Formula.
From rk.md
Arterial Blood Gas (ABG) Interpretation Determining Acidosis and Mixed Gas Formula The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. When they do so, they. Mixed Gas Formula.
From byjus.com
32.One way of writing the equation of state for real gas is PV=R[1+B Mixed Gas Formula \ (p_0\) is not to be confused with the constant \ (p^o\).) Mixture of gases are common in many. (we denote the common initial pressure by “\ (p_0\)”. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is. Mixed Gas Formula.
From www.youtube.com
[7] finding density of a mixture YouTube Mixed Gas Formula (we denote the common initial pressure by “\ (p_0\)”. \ (p_0\) is not to be confused with the constant \ (p^o\).) Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. When they. Mixed Gas Formula.
From flaringmethanetoolkit.com
Composition Measure Density and Infer Gas Composition Methane Mixed Gas Formula Mixture of gases are common in many. (we denote the common initial pressure by “\ (p_0\)”. \ (p_0\) is not to be confused with the constant \ (p^o\).) By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a. Mixed Gas Formula.
From physics.stackexchange.com
thermodynamics How to derive Dalton's law of partial pressure for a Mixed Gas Formula By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. Mixture of gases are common in many. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed. Mixed Gas Formula.
From www.slideshare.net
Mixed Gas Law Problems Mixed Gas Formula To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. When they do so, they become a solution—a homogeneous mixture. Mixture of gases are common in. Mixed Gas Formula.
From chemistryguru.com.sg
Ideal Gas Law and Applications Mixed Gas Formula Mixture of gases are common in many. \ (p_0\) is not to be confused with the constant \ (p^o\).) According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. (we denote the common initial pressure by “\ (p_0\)”. One of the properties of gases is that they mix. Mixed Gas Formula.
From www.studypool.com
SOLUTION 10chap mixed gas laws worksheet Studypool Mixed Gas Formula (we denote the common initial pressure by “\ (p_0\)”. \ (p_0\) is not to be confused with the constant \ (p^o\).) The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of. Mixed Gas Formula.
From byjus.com
Different forms of ideal gas equation Mixed Gas Formula When they do so, they become a solution—a homogeneous mixture. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. \ (p_0\) is not to be. Mixed Gas Formula.
From www.youtube.com
Gas Density & Average Molar Mass of a Gaseous Mixture, Mole Fraction Mixed Gas Formula (we denote the common initial pressure by “\ (p_0\)”. One of the properties of gases is that they mix with each other. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases. Mixed Gas Formula.
From www.straightanursingstudent.com
Mixed up about mixed gas? Straight A Nursing Mixed Gas Formula Mixture of gases are common in many. (we denote the common initial pressure by “\ (p_0\)”. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are. Mixed Gas Formula.
From studylib.net
Fluid Dynamics 1I 1 Mixed Gas Formula By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. \ (p_0\) is not to be confused with the constant \ (p^o\).) When they do so, they become a solution—a homogeneous mixture. According to. Mixed Gas Formula.
From www.expii.com
Combined Gas Law — Overview & Calculations Expii Mixed Gas Formula By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. When they do so, they. Mixed Gas Formula.
From brainly.in
density of gaseous mixture A and b from percentage volume is given by Mixed Gas Formula (we denote the common initial pressure by “\ (p_0\)”. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. When they do so, they become a solution—a homogeneous mixture. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. One of the properties of gases is. Mixed Gas Formula.
From www.chegg.com
The ideal gas law relates the pressure, density, and Mixed Gas Formula One of the properties of gases is that they mix with each other. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. (we denote the common initial pressure by “\ (p_0\)”. \ (p_0\) is not to be confused with the constant \ (p^o\).) According to dalton’s law of partial pressures,. Mixed Gas Formula.
From www.toppr.com
At constant volume and temperature conditions, the rates of diffusion Mixed Gas Formula \ (p_0\) is not to be confused with the constant \ (p^o\).) (we denote the common initial pressure by “\ (p_0\)”. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the. Mixed Gas Formula.
From www.linstitute.net
IB DP Chemistry SL复习笔记1.2.3 Avogadro's Law & Molar Gas Volume翰林国际教育 Mixed Gas Formula By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. The combined. Mixed Gas Formula.
From byjus.com
21. Mean free path of the gas molecules depends onits pressure as Mixed Gas Formula According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. \ (p_0\) is not to be confused with the constant \ (p^o\).) One of the properties of gases is that they mix with each other. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant. Mixed Gas Formula.
From studylib.net
Mixed Gas Law Problems Mixed Gas Formula Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. The combined gas law expresses the relationship between the. Mixed Gas Formula.
From worksheetzone.org
Mixed Gas Laws Worksheet Worksheet Mixed Gas Formula When they do so, they become a solution—a homogeneous mixture. One of the properties of gases is that they mix with each other. Mixture of gases are common in many. (we denote the common initial pressure by “\ (p_0\)”. \ (p_0\) is not to be confused with the constant \ (p^o\).) According to dalton’s law of partial pressures, the total. Mixed Gas Formula.
From www.youtube.com
Combined Gas Law Formula (Chemistry) YouTube Mixed Gas Formula By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. To see how mole fractions. Mixed Gas Formula.
From studylib.net
Mixed Gas Law Worksheet Answers available at end Name (show Mixed Gas Formula When they do so, they become a solution—a homogeneous mixture. Mixture of gases are common in many. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. By convention,. Mixed Gas Formula.
From www.showme.com
Mixed Gas Laws Examples11/11 Science ShowMe Mixed Gas Formula (we denote the common initial pressure by “\ (p_0\)”. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. When they do so, they become a solution—a homogeneous mixture. The combined gas law. Mixed Gas Formula.
From mmerevise.co.uk
The Ideal Gas Equation MME Mixed Gas Formula Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. One of the properties of gases is that they mix with each other. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. Mixture of gases are common in many. The combined gas. Mixed Gas Formula.
From www.slideserve.com
PPT The Combined and Ideal Gas Laws PowerPoint Presentation, free Mixed Gas Formula To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. Mixture of gases are common in many. According to dalton’s law of partial pressures, the total. Mixed Gas Formula.
From www.studypool.com
SOLUTION Gas laws key worksheet with answer Studypool Mixed Gas Formula By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. (we denote the common initial pressure by “\ (p_0\)”. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density.. Mixed Gas Formula.
From www.umpgas.com
Jual Mixed Gas, Gas Mixtures, Harga Mixed Gas, Distributor Mixed Gas Mixed Gas Formula The combined gas law expresses the relationship between the pressure, volume, and absolute temperature of a fixed amount of. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). (we denote the common initial pressure by “\ (p_0\)”. One of the properties of gases is. Mixed Gas Formula.
From physics.stackexchange.com
theory Mean free path for gas mixture Physics Stack Exchange Mixed Gas Formula One of the properties of gases is that they mix with each other. According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\).. Mixed Gas Formula.
From www.youtube.com
Gas Law Formulas and Equations College Chemistry Study Guide YouTube Mixed Gas Formula To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. One of the properties of gases is that they mix with each other.. Mixed Gas Formula.
From www.youtube.com
Calculate Liquid Density of a Mixture YouTube Mixed Gas Formula By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which the two gases initially occupy separate containers, but are both at a common pressure and temperature. To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas. Mixed Gas Formula.
From www.walmart.com
Mixed Gas Mixed Gas Formula To see how mole fractions can help us understand the properties of gas mixtures, let’s evaluate the ratio of the pressure of a gas \(a\). Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. By convention, the process of mixing two gases, call them \ (a\) and \ (b\), is the process in which. Mixed Gas Formula.
From sciencenotes.org
Combined Gas Law Definition, Formula, Examples Mixed Gas Formula According to dalton’s law of partial pressures, the total pressure exerted by the mixture of gases is the sum of the partial. (we denote the common initial pressure by “\ (p_0\)”. Mixture of gases are common in many. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. When they do so, they become a. Mixed Gas Formula.
From www.studocu.com
Mixed Gas Laws Worksheet Grey Mixed Gas Laws Worksheet How many moles Mixed Gas Formula One of the properties of gases is that they mix with each other. \ (p_0\) is not to be confused with the constant \ (p^o\).) When they do so, they become a solution—a homogeneous mixture. (we denote the common initial pressure by “\ (p_0\)”. Mixture of gases are common in many. Gas mixtures and the ideal gas law, mass calculations,. Mixed Gas Formula.
From drcalef.com
The Combined Gas Law Pressure, Volume, and Temperature Mixed Gas Formula Mixture of gases are common in many. Gas mixtures and the ideal gas law, mass calculations, the individual gas constant and density. When they do so, they become a solution—a homogeneous mixture. \ (p_0\) is not to be confused with the constant \ (p^o\).) One of the properties of gases is that they mix with each other. According to dalton’s. Mixed Gas Formula.