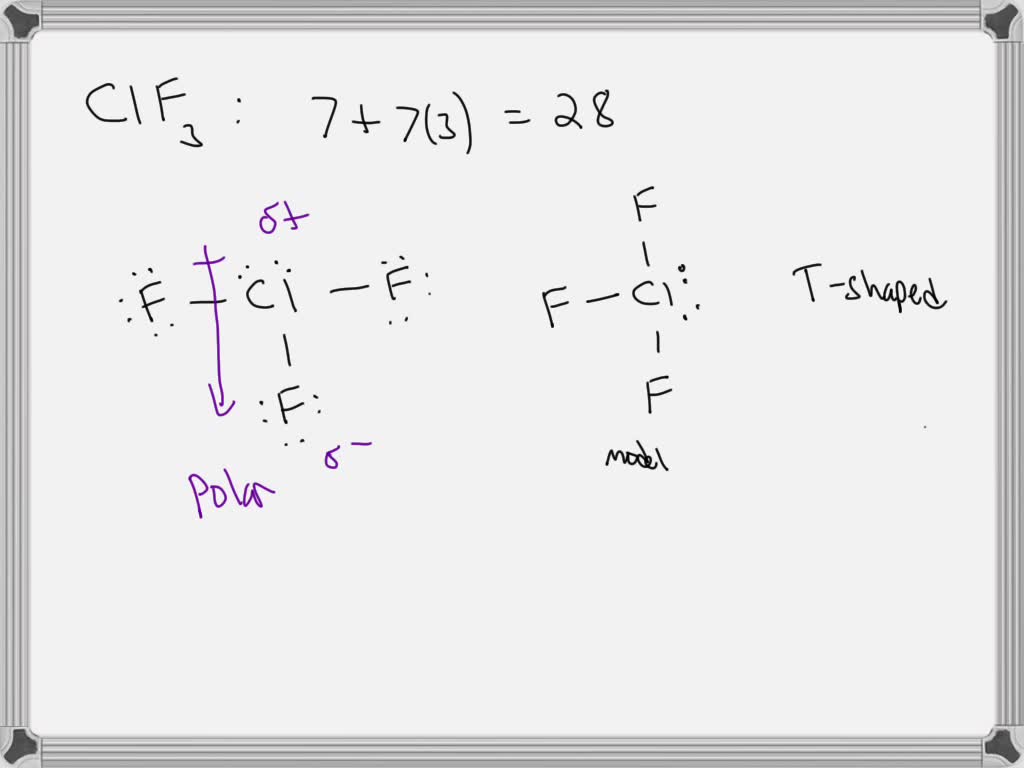Chlorine Trifluoride . See a video of clf3 reacting. Find its molecular formula, average mass,. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. Learn about its history, properties, uses, and dangers in this article. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. This colourless, poisonous, corrosive and very reactive. chlorine trifluoride is the chemical compound with the formula clf 3.
from www.numerade.com
This colourless, poisonous, corrosive and very reactive. chlorine trifluoride is the chemical compound with the formula clf 3. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. Find its molecular formula, average mass,. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. See a video of clf3 reacting.
SOLVED 5) Chlorine trifluoride has two sets of lone pairs of electrons
Chlorine Trifluoride — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. See a video of clf3 reacting. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. Learn about its history, properties, uses, and dangers in this article. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. chlorine trifluoride is the chemical compound with the formula clf 3. This colourless, poisonous, corrosive and very reactive. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. Find its molecular formula, average mass,. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent.
From
Chlorine Trifluoride Find its molecular formula, average mass,. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. chlorine trifluoride is the chemical compound with the. Chlorine Trifluoride.
From alchetron.com
Chlorine trifluoride Alchetron, The Free Social Encyclopedia Chlorine Trifluoride Learn about its history, properties, uses, and dangers in this article. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. chlorine trifluoride is the chemical compound with the formula clf 3. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds. Chlorine Trifluoride.
From www.youtube.com
ClF3 Chlorine Trifluoride Shape Hybridisation VSEPR Problem Chlorine Trifluoride Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. See a video of clf3 reacting. Find its molecular formula, average mass,. Learn about its history, properties, uses, and dangers in this article. This colourless, poisonous, corrosive and very reactive. chlorine trifluoride is the chemical compound with the formula clf 3. — chlorine trifluoride. Chlorine Trifluoride.
From
Chlorine Trifluoride chlorine trifluoride is the chemical compound with the formula clf 3. This colourless, poisonous, corrosive and very reactive. Learn about its history, properties, uses, and dangers in this article. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. See a video of clf3 reacting. a colorless gas or green liquid with a pungent. Chlorine Trifluoride.
From
Chlorine Trifluoride See a video of clf3 reacting. Learn about its history, properties, uses, and dangers in this article. chlorine trifluoride is the chemical compound with the formula clf 3. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising. Chlorine Trifluoride.
From www.youtube.com
ClF3 Molecular Geometry, Bond Angles & Electron Geometry (Chlorine Chlorine Trifluoride Learn about its history, properties, uses, and dangers in this article. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. chlorine trifluoride is the chemical compound with the formula clf 3. chemspider provides information. Chlorine Trifluoride.
From www.shutterstock.com
14 Chlorine Trifluoride Stock Vectors and Vector Art Shutterstock Chlorine Trifluoride learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. Find its molecular formula, average mass,. chlorine trifluoride is the chemical compound with the formula clf 3. See a video of clf3 reacting. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful. Chlorine Trifluoride.
From
Chlorine Trifluoride a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. chlorine trifluoride is the chemical compound with the formula clf 3. Learn about its history, properties, uses, and dangers in this article. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire. Chlorine Trifluoride.
From
Chlorine Trifluoride learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. Find its molecular formula, average mass,. Learn about its history, properties, uses, and dangers in this article. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. . Chlorine Trifluoride.
From www.dreamstime.com
Chlorine Trifluoride Dangerous Poisonous Gas in Chemical Glassware Chlorine Trifluoride Find its molecular formula, average mass,. This colourless, poisonous, corrosive and very reactive. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. chlorine trifluoride is the chemical compound with the formula clf 3. See a video of clf3 reacting. Learn. Chlorine Trifluoride.
From
Chlorine Trifluoride — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. See a video of clf3 reacting. Find its molecular formula, average mass,. Learn about its history, properties, uses, and dangers in this article. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. This colourless, poisonous, corrosive. Chlorine Trifluoride.
From www.alamy.com
Chlorine gas white background hires stock photography and images Alamy Chlorine Trifluoride a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. This colourless, poisonous, corrosive and very reactive. Find out its chemical and physical properties, preparation methods, uses and. Chlorine Trifluoride.
From
Chlorine Trifluoride See a video of clf3 reacting. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. Learn about its history, properties, uses, and dangers in this article. — learn about chlorine trifluoride, an interhalogen compound with. Chlorine Trifluoride.
From
Chlorine Trifluoride See a video of clf3 reacting. Learn about its history, properties, uses, and dangers in this article. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong. Chlorine Trifluoride.
From
Chlorine Trifluoride — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. This colourless, poisonous, corrosive and very reactive. chlorine trifluoride is the chemical compound with the formula clf 3. Find its molecular formula, average mass,. —. Chlorine Trifluoride.
From
Chlorine Trifluoride — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. chlorine trifluoride is the chemical compound with the formula clf 3. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a. Chlorine Trifluoride.
From
Chlorine Trifluoride a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. Find its molecular formula, average mass,. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can. Chlorine Trifluoride.
From
Chlorine Trifluoride Find its molecular formula, average mass,. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. learn about the. Chlorine Trifluoride.
From www.thebusinessresearchcompany.com
Chlorine Trifluoride Market Growth, Drivers, Analysis And Forecast 2033 Chlorine Trifluoride chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. chlorine trifluoride is the chemical compound with the formula clf 3. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a. Chlorine Trifluoride.
From
Chlorine Trifluoride chlorine trifluoride is the chemical compound with the formula clf 3. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. See a video of clf3 reacting. This colourless, poisonous, corrosive and very reactive. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. — chlorine trifluoride (clf3) is a. Chlorine Trifluoride.
From
Chlorine Trifluoride chlorine trifluoride is the chemical compound with the formula clf 3. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. Learn about its history, properties, uses, and. Chlorine Trifluoride.
From
Chlorine Trifluoride See a video of clf3 reacting. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. This colourless,. Chlorine Trifluoride.
From
Chlorine Trifluoride Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. Learn about its history, properties, uses, and dangers in this article. See a video of clf3 reacting. This colourless, poisonous, corrosive and very reactive. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. learn about the properties and dangers of. Chlorine Trifluoride.
From
Chlorine Trifluoride This colourless, poisonous, corrosive and very reactive. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive. Chlorine Trifluoride.
From www.databridgemarketresearch.com
Chlorine Trifluoride Market Global Industry Trends and Forecast to Chlorine Trifluoride chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. See a video of clf3 reacting. chlorine trifluoride is the chemical compound with the formula clf 3. — learn about chlorine trifluoride, an interhalogen compound. Chlorine Trifluoride.
From www.alamy.com
Chlorine trifluoride is an interhalogen compound with the formula ClF3 Chlorine Trifluoride Find its molecular formula, average mass,. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set. Chlorine Trifluoride.
From www.chemistryworld.com
Chlorine trifluoride Podcast Chemistry World Chlorine Trifluoride Learn about its history, properties, uses, and dangers in this article. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. Find its molecular formula, average mass,. See a video of clf3 reacting.. Chlorine Trifluoride.
From ar.inspiredpencil.com
Chlorine Trifluoride Lewis Structure Chlorine Trifluoride a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. Learn about its history, properties, uses, and dangers in this article. — learn about chlorine trifluoride, an interhalogen compound with the formula clf3, that is a powerful oxidiser and a fluorinating agent. learn about the. Chlorine Trifluoride.
From
Chlorine Trifluoride Learn about its history, properties, uses, and dangers in this article. chlorine trifluoride is the chemical compound with the formula clf 3. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. This colourless, poisonous, corrosive. Chlorine Trifluoride.
From
Chlorine Trifluoride a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. Learn about its history,. Chlorine Trifluoride.
From
Chlorine Trifluoride Find its molecular formula, average mass,. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. This colourless, poisonous, corrosive and very reactive. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. Learn about its history, properties, uses, and dangers in this article. chlorine trifluoride. Chlorine Trifluoride.
From www.redbubble.com
"Chlorine Trifluoride ClF3" Sticker by Zeeph Redbubble Chlorine Trifluoride This colourless, poisonous, corrosive and very reactive. Learn about its history, properties, uses, and dangers in this article. Find its molecular formula, average mass,. chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. a colorless. Chlorine Trifluoride.
From www.wikiwand.com
Chlorine trifluoride oxide Wikiwand Chlorine Trifluoride Find its molecular formula, average mass,. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. Learn about its history, properties, uses, and dangers in this article. See a video of clf3 reacting. chlorine trifluoride is the chemical compound with the formula clf 3. Find out. Chlorine Trifluoride.
From
Chlorine Trifluoride chemspider provides information on chlorine trifluoride, a chemical compound with the formula clf3. See a video of clf3 reacting. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. a colorless gas or green liquid with a pungent odor, chlorine trifluoride is a highly reactive oxidant and a strong halogenating agent. — learn. Chlorine Trifluoride.
From
Chlorine Trifluoride — chlorine trifluoride (clf3) is a powerful oxidizing agent that can set fire to glass, sand, and water. learn about the properties and dangers of chlorine trifluoride (clf3), one of the most reactive oxidising compounds known. Find out its chemical and physical properties, preparation methods, uses and frequently asked questions. chlorine trifluoride is the chemical compound with. Chlorine Trifluoride.