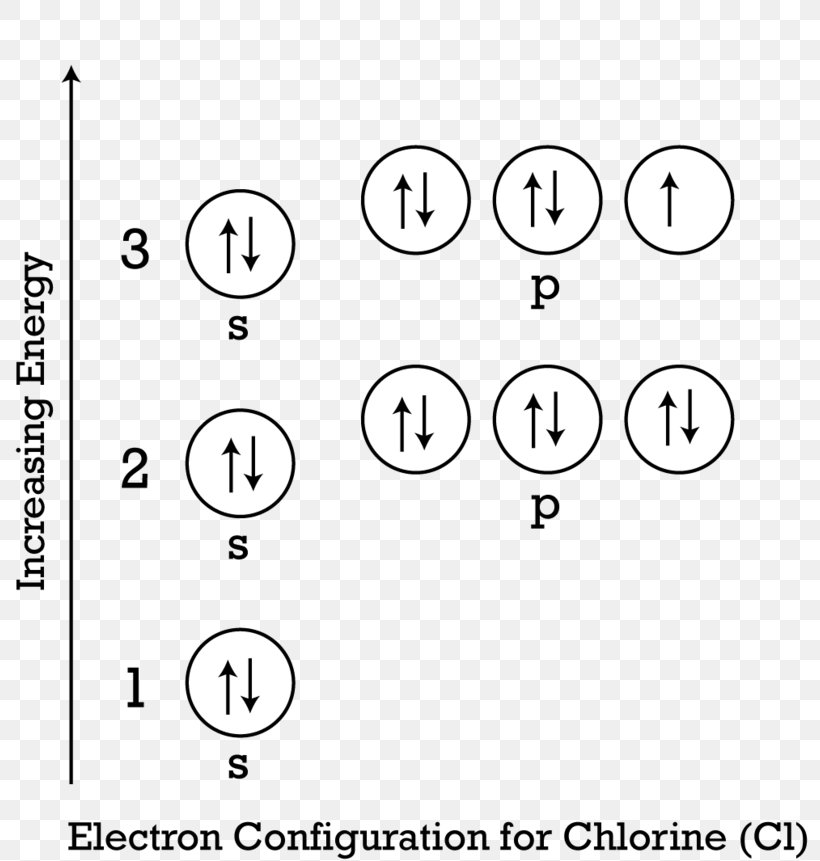
Valence Electrons Do Chlorine . The first is to use the periodic table to figure out how many electrons chlorine. Calcium would have two valence electrons, since it is in group iia (group 2). 119 rows september 1, 2024 by jay. There are two ways to find the number of valence electrons in chlorine (cl). Chlorine is in group viia (group 17), so it would have seven valence electrons. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine has 7 valence electrons. This makes chlorine a cl−1.
from favpng.com
This makes chlorine a cl−1. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. Chlorine has 7 valence electrons. 119 rows september 1, 2024 by jay. It needs one electron to make it stable at 8 electrons in its valence shells. Calcium would have two valence electrons, since it is in group iia (group 2). 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The first is to use the periodic table to figure out how many electrons chlorine. There are two ways to find the number of valence electrons in chlorine (cl). Chlorine is in group viia (group 17), so it would have seven valence electrons.
Electron Configuration Aufbau Principle Valence Electron Chlorine, PNG
Valence Electrons Do Chlorine Calcium would have two valence electrons, since it is in group iia (group 2). Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. This makes chlorine a cl−1. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. There are two ways to find the number of valence electrons in chlorine (cl). Chlorine is in group viia (group 17), so it would have seven valence electrons. Calcium would have two valence electrons, since it is in group iia (group 2). The first is to use the periodic table to figure out how many electrons chlorine. 119 rows september 1, 2024 by jay.
From byjus.com
Find out the number of valence electrons present in chlorine. Valence Electrons Do Chlorine It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine has 7 valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine is in group viia (group 17), so it would have seven valence electrons. There are. Valence Electrons Do Chlorine.
From www.youtube.com
How to find Protons & Electrons for the Chloride ion (Cl) YouTube Valence Electrons Do Chlorine The first is to use the periodic table to figure out how many electrons chlorine. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. Chlorine is in group viia (group 17), so it would have seven valence electrons. 119 rows september 1, 2024 by jay. 93 rows you may assume. Valence Electrons Do Chlorine.
From valenceelectrons.com
How to Find the Valence Electrons for ClF3 (Chlorine Trifluoride)? Valence Electrons Do Chlorine Chlorine is in group viia (group 17), so it would have seven valence electrons. This makes chlorine a cl−1. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Calcium would have two valence electrons, since it is in group iia (group 2). There are two. Valence Electrons Do Chlorine.
From www.britannica.com
ionic bond Definition, Properties, Examples, & Facts Britannica Valence Electrons Do Chlorine As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. 119 rows. Valence Electrons Do Chlorine.
From periodictable.me
Chlorine Electron Configuration (Cl) with Orbital Diagram Valence Electrons Do Chlorine As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine has 7 valence electrons. Calcium would have two valence electrons, since it is in group iia. Valence Electrons Do Chlorine.
From valenceelectrons.com
Protons, Neutrons, Electrons for Chlorine (Cl, Cl) Valence Electrons Do Chlorine It needs one electron to make it stable at 8 electrons in its valence shells. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. There are two ways to find the number of valence electrons in chlorine (cl). 119 rows september 1, 2024 by jay.. Valence Electrons Do Chlorine.
From www.youtube.com
Chlorine Electron Configuration YouTube Valence Electrons Do Chlorine There are two ways to find the number of valence electrons in chlorine (cl). Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It needs one electron to make it stable. Valence Electrons Do Chlorine.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Valence Electrons Do Chlorine There are two ways to find the number of valence electrons in chlorine (cl). This makes chlorine a cl−1. 119 rows september 1, 2024 by jay. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. The first is to use the periodic table to figure out how many electrons chlorine.. Valence Electrons Do Chlorine.
From topblogtenz.com
Chlorine Orbital diagram, Electron configuration, and Valence electrons Valence Electrons Do Chlorine The first is to use the periodic table to figure out how many electrons chlorine. Calcium would have two valence electrons, since it is in group iia (group 2). As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. 119 rows september 1, 2024 by jay. Chlorine has 7 valence electrons.. Valence Electrons Do Chlorine.
From www.animalia-life.club
Electron Configuration For Chlorine Valence Electrons Do Chlorine It needs one electron to make it stable at 8 electrons in its valence shells. The first is to use the periodic table to figure out how many electrons chlorine. There are two ways to find the number of valence electrons in chlorine (cl). 119 rows september 1, 2024 by jay. This makes chlorine a cl−1. Chlorine has 7 valence. Valence Electrons Do Chlorine.
From slideplayer.com
Atom Structure White Board Practice. ppt download Valence Electrons Do Chlorine Calcium would have two valence electrons, since it is in group iia (group 2). There are two ways to find the number of valence electrons in chlorine (cl). This makes chlorine a cl−1. It needs one electron to make it stable at 8 electrons in its valence shells. The first is to use the periodic table to figure out how. Valence Electrons Do Chlorine.
From utedzz.blogspot.com
Periodic Table Chlorine Atomic Number Periodic Table Timeline Valence Electrons Do Chlorine Chlorine has 7 valence electrons. The first is to use the periodic table to figure out how many electrons chlorine. There are two ways to find the number of valence electrons in chlorine (cl). 119 rows september 1, 2024 by jay. It needs one electron to make it stable at 8 electrons in its valence shells. Calcium would have two. Valence Electrons Do Chlorine.
From material-properties.org
Chlorine Periodic Table and Atomic Properties Valence Electrons Do Chlorine The first is to use the periodic table to figure out how many electrons chlorine. Calcium would have two valence electrons, since it is in group iia (group 2). It needs one electron to make it stable at 8 electrons in its valence shells. There are two ways to find the number of valence electrons in chlorine (cl). Chlorine is. Valence Electrons Do Chlorine.
From commons.wikimedia.org
FileElectron shell 017 chlorine.png Wikimedia Commons Valence Electrons Do Chlorine 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Chlorine has 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. 119 rows september 1, 2024 by jay. Calcium would have two valence electrons, since it is in. Valence Electrons Do Chlorine.
From www.youtube.com
How many valence electrons are in a chlorine atom YouTube Valence Electrons Do Chlorine Chlorine is in group viia (group 17), so it would have seven valence electrons. This makes chlorine a cl−1. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. Calcium would have two valence electrons, since it is in group iia (group 2). 93 rows you may assume the valences of. Valence Electrons Do Chlorine.
From elchoroukhost.net
Chlorine Periodic Table Electrons Elcho Table Valence Electrons Do Chlorine Calcium would have two valence electrons, since it is in group iia (group 2). The first is to use the periodic table to figure out how many electrons chlorine. It needs one electron to make it stable at 8 electrons in its valence shells. 119 rows september 1, 2024 by jay. There are two ways to find the number of. Valence Electrons Do Chlorine.
From www.researchgate.net
hypochlorous acid Chlorine has 7 valence electrons, oxygen has 6 Valence Electrons Do Chlorine 119 rows september 1, 2024 by jay. This makes chlorine a cl−1. Chlorine has 7 valence electrons. It needs one electron to make it stable at 8 electrons in its valence shells. Chlorine is in group viia (group 17), so it would have seven valence electrons. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s. Valence Electrons Do Chlorine.
From valenceelectrons.com
Electron Configuration for Chlorine (Cl) Full Explanation Valence Electrons Do Chlorine The first is to use the periodic table to figure out how many electrons chlorine. This makes chlorine a cl−1. Calcium would have two valence electrons, since it is in group iia (group 2). It needs one electron to make it stable at 8 electrons in its valence shells. As another example, an element like chlorine (1s 2 2s 2. Valence Electrons Do Chlorine.
From thvinhtuy.edu.vn
Lewis structure Chlorine Valence electron Diagram, 18, chemical Element Valence Electrons Do Chlorine 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. Chlorine is in group viia (group 17), so it would have seven valence electrons. There are two. Valence Electrons Do Chlorine.
From chemistry291.blogspot.com
How Many Valence Electrons Does chlorine Have?number of valence Valence Electrons Do Chlorine 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. This makes chlorine a cl−1. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. Chlorine is in group viia (group 17), so it would have seven. Valence Electrons Do Chlorine.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Valence Electrons Do Chlorine 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It needs one electron to make it stable at 8 electrons in its valence shells. 119 rows september 1, 2024 by jay. Chlorine has 7 valence electrons. As another example, an element like chlorine (1s 2. Valence Electrons Do Chlorine.
From brokeasshome.com
Periodic Table Chlorine Electrons Valence Electrons Do Chlorine 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The first is to use the periodic table to figure out how many electrons chlorine. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. 119 rows. Valence Electrons Do Chlorine.
From www.alamy.com
Chlorine (Cl). Diagram of the nuclear composition, electron Valence Electrons Do Chlorine 119 rows september 1, 2024 by jay. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. There are two ways to find the number of valence electrons in chlorine (cl). Chlorine has 7 valence electrons. It needs one electron to make it stable at 8. Valence Electrons Do Chlorine.
From www.vectorstock.com
Diagram representation of the element chlorine Vector Image Valence Electrons Do Chlorine Chlorine has 7 valence electrons. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. 119 rows september 1, 2024 by jay. It needs one electron to make it stable at 8 electrons in its valence shells. Calcium would have two valence electrons, since it is in group iia (group 2).. Valence Electrons Do Chlorine.
From www.slideserve.com
PPT Electron Energy Levels and Configurations PowerPoint Presentation Valence Electrons Do Chlorine 119 rows september 1, 2024 by jay. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. The first is to use the periodic table to figure out how many electrons chlorine. There are two ways to find the number of valence electrons in chlorine (cl).. Valence Electrons Do Chlorine.
From www.teachoo.com
Uses of Metals and Non Metals [in Daily life] Teachoo Concepts Valence Electrons Do Chlorine Chlorine is in group viia (group 17), so it would have seven valence electrons. The first is to use the periodic table to figure out how many electrons chlorine. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. There are two ways to find the. Valence Electrons Do Chlorine.
From valenceelectrons.com
How Many Valence Electrons Does Chlorine (Cl) Have? Valence Electrons Do Chlorine As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Calcium would have. Valence Electrons Do Chlorine.
From www.chegg.com
Solved What is the electron configuration for Cl, chlorine, Valence Electrons Do Chlorine The first is to use the periodic table to figure out how many electrons chlorine. Chlorine is in group viia (group 17), so it would have seven valence electrons. This makes chlorine a cl−1. There are two ways to find the number of valence electrons in chlorine (cl). It needs one electron to make it stable at 8 electrons in. Valence Electrons Do Chlorine.
From www.youtube.com
How to Draw the Lewis Structure for ClO2 (Chlorine dioxide) YouTube Valence Electrons Do Chlorine Chlorine has 7 valence electrons. Chlorine is in group viia (group 17), so it would have seven valence electrons. The first is to use the periodic table to figure out how many electrons chlorine. Calcium would have two valence electrons, since it is in group iia (group 2). There are two ways to find the number of valence electrons in. Valence Electrons Do Chlorine.
From byjus.com
In the electron dot structure, the valence shell electrons are Valence Electrons Do Chlorine 119 rows september 1, 2024 by jay. Calcium would have two valence electrons, since it is in group iia (group 2). This makes chlorine a cl−1. There are two ways to find the number of valence electrons in chlorine (cl). Chlorine is in group viia (group 17), so it would have seven valence electrons. 93 rows you may assume the. Valence Electrons Do Chlorine.
From www.ck12.org
Valence Electrons CK12 Foundation Valence Electrons Do Chlorine 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. Calcium would have two valence electrons, since it is in group iia (group 2). Chlorine has 7 valence electrons. There are two ways to find the number of valence electrons in chlorine (cl). It needs one. Valence Electrons Do Chlorine.
From pixels.com
Chlorine Electron Configuration Photograph by Valence Electrons Do Chlorine There are two ways to find the number of valence electrons in chlorine (cl). It needs one electron to make it stable at 8 electrons in its valence shells. Calcium would have two valence electrons, since it is in group iia (group 2). As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5). Valence Electrons Do Chlorine.
From www.youtube.com
How many valence electrons does chlorine have?How to find the valence Valence Electrons Do Chlorine Chlorine is in group viia (group 17), so it would have seven valence electrons. This makes chlorine a cl−1. The first is to use the periodic table to figure out how many electrons chlorine. Chlorine has 7 valence electrons. 119 rows september 1, 2024 by jay. There are two ways to find the number of valence electrons in chlorine (cl).. Valence Electrons Do Chlorine.
From womackthille.blogspot.com
Expanded Electron Configuration of Chlorine Womack Thille Valence Electrons Do Chlorine Chlorine is in group viia (group 17), so it would have seven valence electrons. As another example, an element like chlorine (1s 2 2s 2 2p 6 3s 2 3p 5) will have. This makes chlorine a cl−1. The first is to use the periodic table to figure out how many electrons chlorine. Calcium would have two valence electrons, since. Valence Electrons Do Chlorine.
From favpng.com
Electron Configuration Aufbau Principle Valence Electron Chlorine, PNG Valence Electrons Do Chlorine 93 rows you may assume the valences of the chemical elements—the number of electrons with which an atom will bond or form—are those that. It needs one electron to make it stable at 8 electrons in its valence shells. 119 rows september 1, 2024 by jay. There are two ways to find the number of valence electrons in chlorine (cl).. Valence Electrons Do Chlorine.