Lead Chloride Elements Combined . If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements.
from www.911metallurgist.com
when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from.
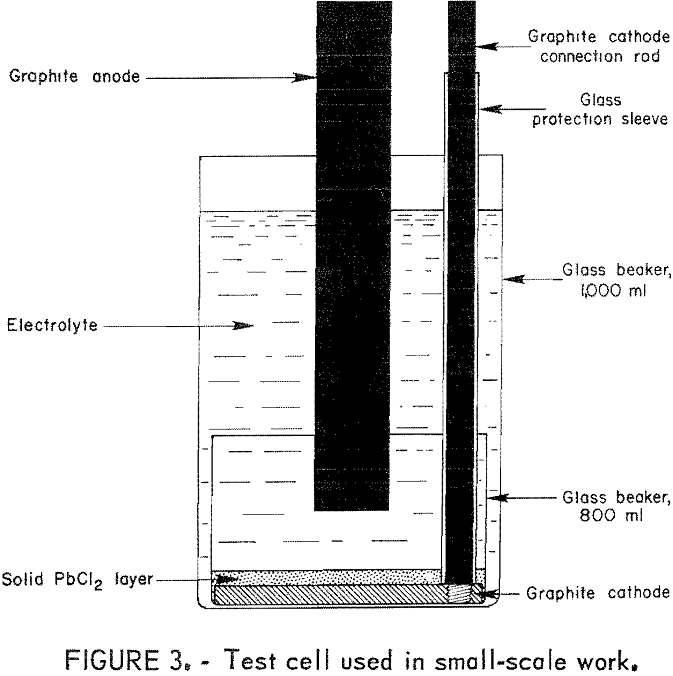
Lead Chloride Electrolysis
Lead Chloride Elements Combined learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If you're behind a web filter,. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements.
From www.sciencesource.com
Lead (II) Chloride and Potassium Chrom Stock Image Science Source Images Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. when forming ions, elements. Lead Chloride Elements Combined.
From www.wikidoc.org
Lead wikidoc Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. if. Lead Chloride Elements Combined.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about. Lead Chloride Elements Combined.
From wisc.pb.unizin.org
Acids, Bases, Neutralization, and GasForming Reactions (M3Q34) UWMadison Chemistry 103/104 Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. if. Lead Chloride Elements Combined.
From www.youtube.com
CHEM TUTORIAL Combining Elements Into (YEAR 1 CHEM) YouTube Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. If you're behind a web filter,. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. when forming ions, elements typically gain or lose the minimum number of electrons necessary to. Lead Chloride Elements Combined.
From www.vecteezy.com
Lead symbol. Chemical element of the periodic table. Vector illustration. 13052123 Vector Art at Lead Chloride Elements Combined learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If you're behind a. Lead Chloride Elements Combined.
From www.gkseries.com
Lead chloride is Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn. Lead Chloride Elements Combined.
From www.numerade.com
SOLVED Aqueous solutions of lead (Il) acetate and calcium chloride are combined. Lead (II Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. learn. Lead Chloride Elements Combined.
From www.nagwa.com
Question Video Identifying the Diagram Representing How Chlorine Molecules Combine with Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If you're behind a web filter,. learn how sodium and chlorine combine to form sodium chloride (salt), a. Lead Chloride Elements Combined.
From www.indiamart.com
Lead Chloride at best price in Mumbai by Yogi Dye Chem Industries ID 2341290833 Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. if you're seeing this message, it means we're having trouble loading external resources on our website. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn about. Lead Chloride Elements Combined.
From www.youtube.com
How to write Molecular formula of lead chloride Chemical formula of Lead chloride YouTube Lead Chloride Elements Combined If you're behind a web filter,. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. if you're seeing this message, it means we're having trouble loading external resources on our website. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full. Lead Chloride Elements Combined.
From www.slideserve.com
PPT Number One Sodium chloride solution + lead (II) acetate solutions react PowerPoint Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If you're. Lead Chloride Elements Combined.
From www.sliderbase.com
Naming compounds and ions Presentation Chemistry Lead Chloride Elements Combined learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. . Lead Chloride Elements Combined.
From www.numerade.com
When lead chloride (PbClz) is placed in otherwise pure water; enough dissolves such that the Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii). Lead Chloride Elements Combined.
From fphoto.photoshelter.com
science chemistry precipitation reaction lead (ii) chloride Fundamental Photographs The Art Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. if you're seeing this message, it means we're having trouble loading external resources on our website. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about. Lead Chloride Elements Combined.
From www.sliderbase.com
Ionic Bonding Presentation Chemistry Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. If you're behind a web filter,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve. Lead Chloride Elements Combined.
From www.toppr.com
5. Write a balanced chemical equations the following chemical reactions (a) Hydrogen Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter,. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii). Lead Chloride Elements Combined.
From questions.kunduz.com
2. (5 marks ) Determine whether a precip... Chemistry Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If. Lead Chloride Elements Combined.
From www.researchgate.net
Crystal structure of 1. a) Three types of lead chloride polyhedra,... Download Scientific Diagram Lead Chloride Elements Combined If you're behind a web filter,. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound. Lead Chloride Elements Combined.
From guides.byjusweb.com
Lead II Chloride Formula Definition, Concepts and Examples Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn how sodium. Lead Chloride Elements Combined.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. If you're. Lead Chloride Elements Combined.
From www.vectorstock.com
Periodic table element chlorine icon Royalty Free Vector Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. if you're seeing this message, it means we're having trouble loading external resources on our website. when forming ions, elements. Lead Chloride Elements Combined.
From www.dreamstime.com
Chemical element Lead stock illustration. Illustration of research 129791907 Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If you're behind a web filter,. when forming ions, elements typically gain or lose the minimum number of electrons necessary to. Lead Chloride Elements Combined.
From www.chemedx.org
Demonstrating the Colors of Transition Metal Complex Ions Chemical Education Xchange Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. if you're seeing this message, it means we're having trouble loading external resources on our website. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. If you're behind. Lead Chloride Elements Combined.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about. Lead Chloride Elements Combined.
From www.youtube.com
How to write chemical formula Lead IV ChlorideMolecular formula Lead IV Chloride YouTube Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. if. Lead Chloride Elements Combined.
From www.wikidoc.org
Lead wikidoc Lead Chloride Elements Combined when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. . Lead Chloride Elements Combined.
From encyclopedia.pub
Compounds of Lead Encyclopedia MDPI Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. If you're behind a web filter,. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii). Lead Chloride Elements Combined.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn. Lead Chloride Elements Combined.
From chemcraft.su
Lead(II) chloride, 99.5 pure p.a. chemcraft.su Lead Chloride Elements Combined learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. when forming ions, elements typically gain or lose the minimum number of electrons necessary to achieve a full octet. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn. Lead Chloride Elements Combined.
From www.911metallurgist.com
Lead Chloride Electrolysis Lead Chloride Elements Combined learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If you're behind a web filter,. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability, and reactions of carbon, silicon, and lead. Lead Chloride Elements Combined.
From www.youtube.com
Lead(II) Chloride Synthesis (PbCl2) YouTube Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. if you're seeing this message, it means we're having trouble loading external resources on our website. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If you're. Lead Chloride Elements Combined.
From www.youtube.com
Equation for PbCl2 + H2O Lead (II) chloride + Water YouTube Lead Chloride Elements Combined If you're behind a web filter,. if you're seeing this message, it means we're having trouble loading external resources on our website. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride. Lead Chloride Elements Combined.
From www.fishersci.no
Lead(II) chloride, ultra dry, 99.999 (metals basis), Thermo Scientific Chemicals Fisher Lead Chloride Elements Combined if you're seeing this message, it means we're having trouble loading external resources on our website. If you're behind a web filter,. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties. Lead Chloride Elements Combined.
From dir.indiamart.com
Lead Chloride PbCl2 Latest Price, Manufacturers & Suppliers Lead Chloride Elements Combined learn how sodium and chlorine combine to form sodium chloride (salt), a common ionic compound with different properties from. learn about the structures, stability, and reactions of carbon, silicon, and lead tetrachlorides, and lead (ii) chloride. learn about the structures, stability and reactions with water of the tetrachlorides and lead (ii) chloride of group 4 elements. If. Lead Chloride Elements Combined.