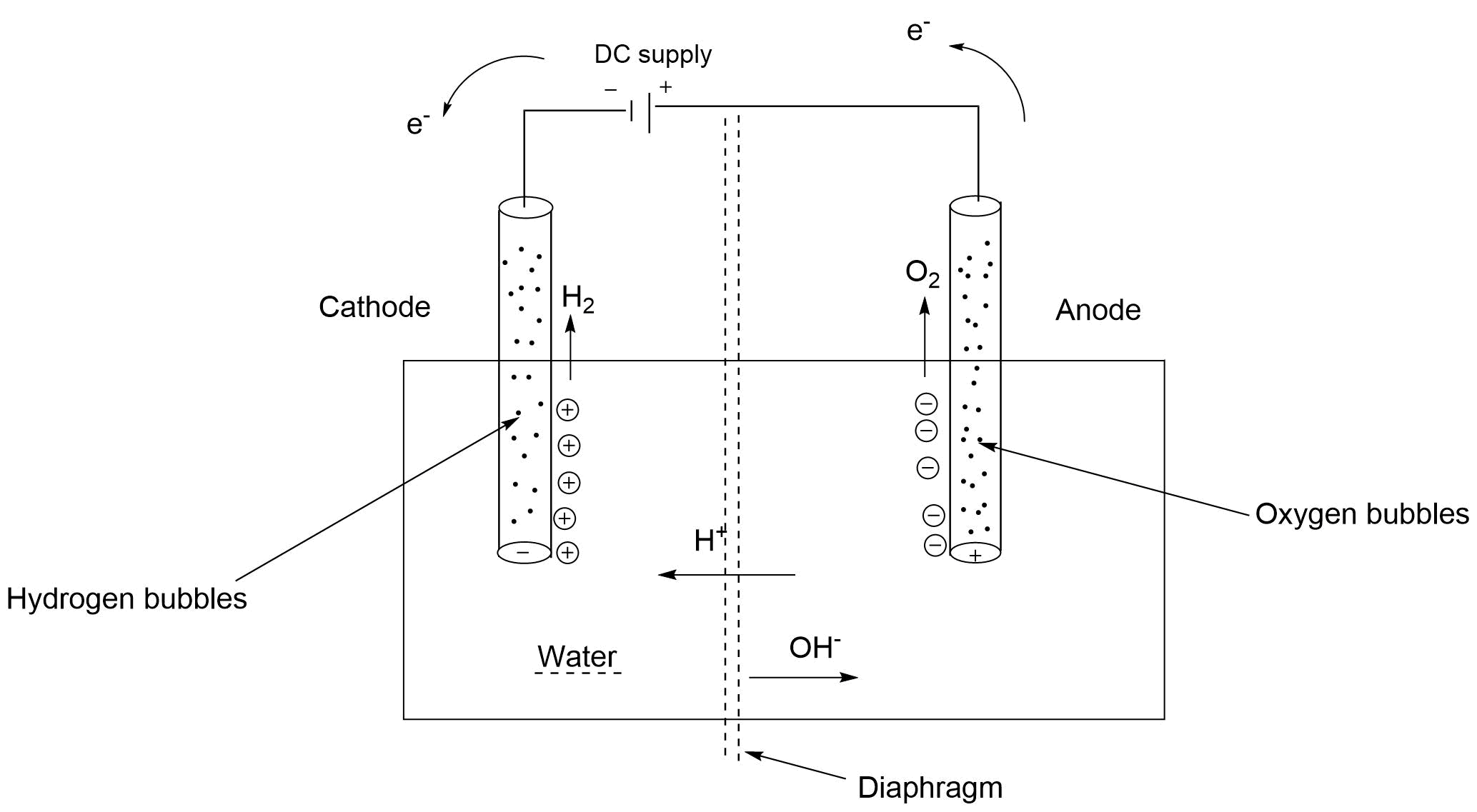
Water Requirement For Electrolysis . The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? Electrolysis can also be used to produce h 2 and o 2 from water. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. At the anode, water is oxidized to oxygen. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential:
from www.vedantu.com
At the anode, water is oxidized to oxygen. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. Electrolysis can also be used to produce h 2 and o 2 from water. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label?
Explain what is meant by electrolysis of water. Write the electrode
Water Requirement For Electrolysis At the anode, water is oxidized to oxygen. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? At the anode, water is oxidized to oxygen. The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Electrolysis can also be used to produce h 2 and o 2 from water.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Water Requirement For Electrolysis The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. At the anode, water is oxidized to oxygen. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water electrolysis is divided into three categories depending on the charge carrier and commercialization. Water Requirement For Electrolysis.
From www.alamy.com
water electrolysis hires stock photography and images Water Requirement For Electrolysis Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: At the anode, water is oxidized to oxygen. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. In practice,. Water Requirement For Electrolysis.
From www.researchgate.net
Configurations for water electrolysis (a) proton exchange membrane Water Requirement For Electrolysis Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: At the anode, water is oxidized to oxygen. In practice,. Water Requirement For Electrolysis.
From enagic-asia.com
The electrolysis process Enagic Kangen Water Water Requirement For Electrolysis Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Electrolysis can also be used to produce h 2 and o 2 from water. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? At the anode, water is oxidized to oxygen. The electrolyte is necessary because pure. Water Requirement For Electrolysis.
From phys.org
A powerful catalyst for electrolysis of water that could help harness Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Electrolysis can also be used to produce h 2 and o 2 from water. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water suitable for electrolysis is commonly called ultrapure, but what is. Water Requirement For Electrolysis.
From byjus.com
Explain the electrolysis of water with the help of diagram. Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Electrolysis can also be used to produce h 2 and o 2 from water. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolysis of water requires a minimum of 237.13 kj of. Water Requirement For Electrolysis.
From www.youtube.com
WATER ELECTROLYSIS DEMONSTRATION WITH EXPLANATION CHEMISTRY GRADE 8 Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. At the anode, water is oxidized to oxygen. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood. Water Requirement For Electrolysis.
From general.chemistrysteps.com
Electrolysis of Water Chemistry Steps Water Requirement For Electrolysis The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Electrolysis can also be used to produce. Water Requirement For Electrolysis.
From www.h2-view.com
Video What are the water requirements for electrolysis? Video H2 View Water Requirement For Electrolysis Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. At the anode, water is oxidized to oxygen. Electrolysis can also be used to produce h 2 and o 2 from water. The. Water Requirement For Electrolysis.
From scienceinfo.com
Electrolysis of Water Definition, Principle, and Applications Water Requirement For Electrolysis The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. Water electrolysis is divided into three categories. Water Requirement For Electrolysis.
From www.yaclass.in
Electrolysis of Water — lesson. Science CBSE, Class 10. Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water suitable for electrolysis is commonly called. Water Requirement For Electrolysis.
From www.mdpi.com
Processes Free FullText CFD Modeling and Experimental Validation Water Requirement For Electrolysis Electrolysis can also be used to produce h 2 and o 2 from water. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water electrolysis is divided into three categories depending on the charge. Water Requirement For Electrolysis.
From ptx-hub.org
Water electrolysis explained the basis for most PowertoX processes Water Requirement For Electrolysis Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? Electrolysis can also be used to produce h 2 and o 2 from water. The electrolysis of water requires a minimum of 237.13 kj of electrical energy input. Water Requirement For Electrolysis.
From www.researchgate.net
H2 generation from seawater electrolysis. a) Direct (one‐step) and Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Electrolysis can also be used to produce h. Water Requirement For Electrolysis.
From www.researchgate.net
Schematic illustration of (a) traditional water electrolysis and (b Water Requirement For Electrolysis The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: At the anode, water is oxidized to oxygen. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolyte. Water Requirement For Electrolysis.
From fixlibrarygedwaaldebx.z21.web.core.windows.net
Circuit Diagram For Electrolysis Of Water Water Requirement For Electrolysis The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. At the anode, water is oxidized to oxygen. The electrolyte is necessary because pure water will not carry enough charge due to. Water Requirement For Electrolysis.
From www.researchgate.net
The fundamental of water electrolysis process. Download Scientific Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Electrolysis can also be used to produce h 2 and o 2 from water. At the anode, water is oxidized to oxygen. The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions.. Water Requirement For Electrolysis.
From www.priyamstudycentre.com
Water Electrolysis Energy Requirement, Electrolyzer, Electrolyte Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? Electrolysis can also be used to produce h 2 and o 2 from water. Water electrolysis is divided into three categories depending on. Water Requirement For Electrolysis.
From encyclopedia.pub
Overview of Water Electrochemistry Encyclopedia MDPI Water Requirement For Electrolysis Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? At the anode, water is oxidized to oxygen. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Electrolysis can also be used to produce h 2 and o 2 from water. The electrolysis of water requires a. Water Requirement For Electrolysis.
From www.awoe.net
A World Of Energy Water electrolysis Water Requirement For Electrolysis Electrolysis can also be used to produce h 2 and o 2 from water. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolysis of water requires a minimum of 237.13 kj of electrical energy input. Water Requirement For Electrolysis.
From pubs.rsc.org
Principles and implementations of electrolysis systems for water Water Requirement For Electrolysis The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water electrolysis is divided into. Water Requirement For Electrolysis.
From www.sscadda.com
Electrolysis of Water Equation, Diagram and Experiment Water Requirement For Electrolysis Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? At the anode, water is oxidized to oxygen. Electrolysis can also be used to produce h 2 and o 2 from water. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolysis of water requires a. Water Requirement For Electrolysis.
From www.researchgate.net
Mechanism of the twostep alkaline water electrolysis. (a) A Water Requirement For Electrolysis Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. The electrolyte is necessary because pure water will not carry. Water Requirement For Electrolysis.
From www.researchgate.net
Schematic of water electrolysis system. Download Scientific Diagram Water Requirement For Electrolysis Electrolysis can also be used to produce h 2 and o 2 from water. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. The electrolyte is necessary because pure water will not carry enough charge due. Water Requirement For Electrolysis.
From vectormine.com
Water electrolysis process, scientific chemistry diagram VectorMine Water Requirement For Electrolysis At the anode, water is oxidized to oxygen. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label?. Water Requirement For Electrolysis.
From morrisclassicalacademy.blogspot.com
Morris Classical Academy Electrolysis of water Water Requirement For Electrolysis Electrolysis can also be used to produce h 2 and o 2 from water. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. At the anode, water is oxidized to oxygen. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: Water suitable for. Water Requirement For Electrolysis.
From www.dreamstime.com
Fully Labeled Diagram Of The Electrolysis Of Water Stock Illustration Water Requirement For Electrolysis The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. Electrolysis can also be used to produce h 2 and o 2 from water. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. At the anode, water is oxidized to oxygen.. Water Requirement For Electrolysis.
From www.chemistrylearner.com
Electrolysis of Water Definition and Equation Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. At the anode, water is. Water Requirement For Electrolysis.
From www.researchgate.net
Performance comparison of three types of water electrolysis Water Requirement For Electrolysis Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. Electrolysis can also be used to produce h 2. Water Requirement For Electrolysis.
From www.vedantu.com
Explain what is meant by electrolysis of water. Write the electrode Water Requirement For Electrolysis Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Electrolysis can also be used to produce h 2 and o 2 from water. The electrolysis of water requires a minimum of 237.13. Water Requirement For Electrolysis.
From www.chemicals.co.uk
What is a Chemical Change? The Chemistry Blog Water Requirement For Electrolysis The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. At the anode, water is oxidized to oxygen. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? Electrolysis can also be used to produce h 2 and o 2 from water. In practice, an. Water Requirement For Electrolysis.
From www.tessshebaylo.com
Alkaline Water Electrolysis Equation Tessshebaylo Water Requirement For Electrolysis Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. At the anode, water is oxidized to oxygen. The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each. Water Requirement For Electrolysis.
From www.hotelsrate.org
Water Electrolysis Equation Modern Home Designs Water Requirement For Electrolysis The electrolyte is necessary because pure water will not carry enough charge due to the lack of ions. Electrolysis can also be used to produce h 2 and o 2 from water. In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water suitable for electrolysis is commonly called ultrapure,. Water Requirement For Electrolysis.
From www.wikihow.com
How to Electrolyse Water 12 Steps (with Pictures) wikiHow Water Requirement For Electrolysis In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. The electrolysis of water requires a minimum of 237.13 kj of electrical energy input to dissociate each mole. Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? At the anode, water is. Water Requirement For Electrolysis.
From www.linkedin.com
Water Electrolysis Different Methods Water Requirement For Electrolysis Water suitable for electrolysis is commonly called ultrapure, but what is to be understood by this label? In practice, an additional voltage, called an overvoltage, must be applied to overcome factors such as a large activation energy. Water electrolysis is divided into three categories depending on the charge carrier and commercialization potential: The electrolyte is necessary because pure water will. Water Requirement For Electrolysis.