What Is The Oxidation Number Of N In Zn No3 2 . To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Zn + 2 hno3 → zn (no3)2 + h2. Zno + 2 hno3 → zn (no3)2 + h2o. The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Enter an equation of a redox chemical reaction and press the balance button. These reactions are accompanied by the hydration of. A net ionic charge can be specified at the end of the. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: The balanced equation will be calculated along with the oxidation. Enter the formula of a chemical compound to find the oxidation number of each element.
from www.slideserve.com
The balanced equation will be calculated along with the oxidation. Zn + 2 hno3 → zn (no3)2 + h2. A net ionic charge can be specified at the end of the. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: Enter an equation of a redox chemical reaction and press the balance button. The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. These reactions are accompanied by the hydration of. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Enter the formula of a chemical compound to find the oxidation number of each element. Zno + 2 hno3 → zn (no3)2 + h2o.
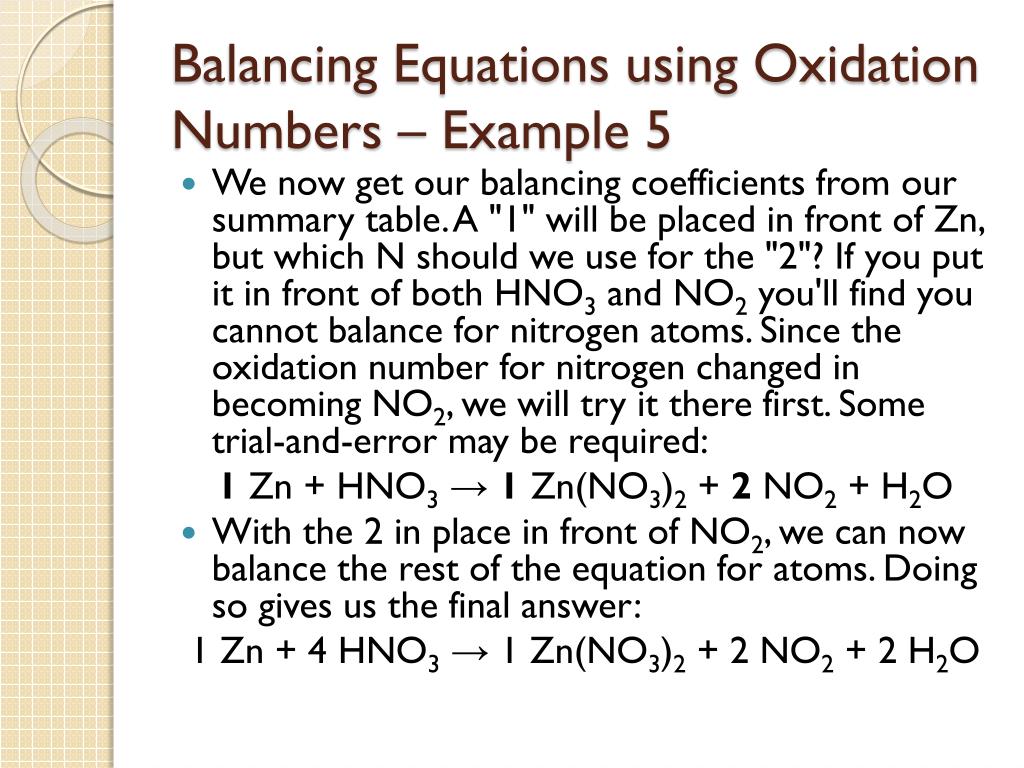
PPT 7.3 Balancing Redox Reactions Using Oxidation Numbers
What Is The Oxidation Number Of N In Zn No3 2 Enter an equation of a redox chemical reaction and press the balance button. Enter an equation of a redox chemical reaction and press the balance button. Zn + 2 hno3 → zn (no3)2 + h2. The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: These reactions are accompanied by the hydration of. Enter the formula of a chemical compound to find the oxidation number of each element. The balanced equation will be calculated along with the oxidation. A net ionic charge can be specified at the end of the. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Zno + 2 hno3 → zn (no3)2 + h2o.
From www.youtube.com
How to find the Oxidation Number for Zn (Zinc) YouTube What Is The Oxidation Number Of N In Zn No3 2 The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: A net ionic charge can be specified at the end of the. Oxidation number calculator is a. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT 7.3 Balancing Redox Reactions Using Oxidation Numbers What Is The Oxidation Number Of N In Zn No3 2 Enter an equation of a redox chemical reaction and press the balance button. Zno + 2 hno3 → zn (no3)2 + h2o. Zn + 2 hno3 → zn (no3)2 + h2. The balanced equation will be calculated along with the oxidation. Enter the formula of a chemical compound to find the oxidation number of each element. Zinc nitrate is usually. What Is The Oxidation Number Of N In Zn No3 2.
From www.youtube.com
Balance the following equation by oxidation number method `Zn+HNO_(3 What Is The Oxidation Number Of N In Zn No3 2 To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: The balanced equation will be calculated along with the oxidation. Zno + 2 hno3 → zn (no3)2 + h2o. Enter the formula of a chemical compound to find the oxidation number of each element. Zinc nitrate is usually prepared by dissolving zinc. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Oxidation Reduction Reactions PowerPoint Presentation, free What Is The Oxidation Number Of N In Zn No3 2 Enter an equation of a redox chemical reaction and press the balance button. These reactions are accompanied by the hydration of. The balanced equation will be calculated along with the oxidation. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Enter the formula of a chemical compound to find the oxidation. What Is The Oxidation Number Of N In Zn No3 2.
From slideplayer.com
OXIDATION AND REDUCTION ppt download What Is The Oxidation Number Of N In Zn No3 2 Zn + 2 hno3 → zn (no3)2 + h2. Zno + 2 hno3 → zn (no3)2 + h2o. The balanced equation will be calculated along with the oxidation. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. These reactions are accompanied by the hydration of. Zinc nitrate is usually prepared by. What Is The Oxidation Number Of N In Zn No3 2.
From www.toppr.com
Complete and balance the given reaction. Zn + NO3^ Zn^2 + + NH4^+ What Is The Oxidation Number Of N In Zn No3 2 The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. The balanced equation will be calculated along with the oxidation. Zno + 2 hno3 → zn (no3)2 + h2o. Enter the formula of a chemical compound to find the oxidation number of each element.. What Is The Oxidation Number Of N In Zn No3 2.
From www.chegg.com
Solved The reaction Zn(s) + Cu(NO3)2(aq) → Zn(NO3)2(aq) + What Is The Oxidation Number Of N In Zn No3 2 The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. These reactions are accompanied by the hydration of. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Enter the formula of a chemical compound to. What Is The Oxidation Number Of N In Zn No3 2.
From www.youtube.com
How to Write the Net Ionic Equation for Zn + Pb(NO3)2 = Zn(NO3)2 + Pb What Is The Oxidation Number Of N In Zn No3 2 Enter the formula of a chemical compound to find the oxidation number of each element. Zn + 2 hno3 → zn (no3)2 + h2. The balanced equation will be calculated along with the oxidation. Enter an equation of a redox chemical reaction and press the balance button. Zno + 2 hno3 → zn (no3)2 + h2o. Oxidation number calculator is. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT 7.3 Balancing Redox Reactions Using Oxidation Numbers What Is The Oxidation Number Of N In Zn No3 2 These reactions are accompanied by the hydration of. The balanced equation will be calculated along with the oxidation. Zno + 2 hno3 → zn (no3)2 + h2o. Zn + 2 hno3 → zn (no3)2 + h2. A net ionic charge can be specified at the end of the. To calculate oxidation numbers of elements in the chemical compound, enter it's. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Electrochemistry Lesson 3 Oxidation Numbers PowerPoint What Is The Oxidation Number Of N In Zn No3 2 Zn + 2 hno3 → zn (no3)2 + h2. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Enter the formula of a chemical compound to find the oxidation number of each element. The balanced equation will be calculated along with the oxidation. The nitrogen in the no 2 ion has. What Is The Oxidation Number Of N In Zn No3 2.
From irvinncesey.blogspot.com
How to Calculate Oxidation Number IrvinnCesey What Is The Oxidation Number Of N In Zn No3 2 A net ionic charge can be specified at the end of the. The balanced equation will be calculated along with the oxidation. Zno + 2 hno3 → zn (no3)2 + h2o. Zn + 2 hno3 → zn (no3)2 + h2. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. The nitrogen. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Oxidation Numbers & Redox Reactions PowerPoint Presentation ID What Is The Oxidation Number Of N In Zn No3 2 The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn No3 2 A net ionic charge can be specified at the end of the. Zn + 2 hno3 → zn (no3)2 + h2. The balanced equation will be calculated along with the oxidation. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: To calculate oxidation numbers of elements in the chemical compound, enter it's. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Chapter 19 Electrochemistry PowerPoint Presentation, free What Is The Oxidation Number Of N In Zn No3 2 Enter the formula of a chemical compound to find the oxidation number of each element. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Zn + 2 hno3 → zn (no3)2 + h2. Enter an equation of a redox chemical reaction and press the balance button. Zno + 2 hno3 →. What Is The Oxidation Number Of N In Zn No3 2.
From www.youtube.com
How To Calculate Oxidation Numbers Basic Introduction YouTube What Is The Oxidation Number Of N In Zn No3 2 Zn + 2 hno3 → zn (no3)2 + h2. A net ionic charge can be specified at the end of the. Enter the formula of a chemical compound to find the oxidation number of each element. The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to. What Is The Oxidation Number Of N In Zn No3 2.
From www.youtube.com
How to find the Oxidation Number for N in NO3 (Nitrate ion) YouTube What Is The Oxidation Number Of N In Zn No3 2 These reactions are accompanied by the hydration of. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: A net ionic charge can be specified at the end of the. Zn + 2 hno3 → zn (no3)2 + h2. Enter an equation of a redox chemical reaction and press the balance button. The. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn No3 2 Zno + 2 hno3 → zn (no3)2 + h2o. Enter an equation of a redox chemical reaction and press the balance button. The balanced equation will be calculated along with the oxidation. Zn + 2 hno3 → zn (no3)2 + h2. These reactions are accompanied by the hydration of. A net ionic charge can be specified at the end of. What Is The Oxidation Number Of N In Zn No3 2.
From brainly.in
What is oxidation number of N in Cu(NO3)2 ? Plz show me how did you What Is The Oxidation Number Of N In Zn No3 2 Enter the formula of a chemical compound to find the oxidation number of each element. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: A net ionic charge can be specified at the end of the. Enter an equation of a redox chemical reaction and press the balance button. Zn + 2. What Is The Oxidation Number Of N In Zn No3 2.
From rex-kluna.blogspot.com
Oxidation Number of N What Is The Oxidation Number Of N In Zn No3 2 Enter an equation of a redox chemical reaction and press the balance button. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. Zno + 2. What Is The Oxidation Number Of N In Zn No3 2.
From www.youtube.com
How to find the Oxidation Numbers for Zn(NO3)2 (Zinc nitrate) YouTube What Is The Oxidation Number Of N In Zn No3 2 Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. The balanced equation will be calculated along with the oxidation. These reactions are accompanied by the hydration of. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: Zno + 2 hno3 → zn (no3)2. What Is The Oxidation Number Of N In Zn No3 2.
From www.sliderbase.com
Oxidation Numbers and names What Is The Oxidation Number Of N In Zn No3 2 A net ionic charge can be specified at the end of the. These reactions are accompanied by the hydration of. Zno + 2 hno3 → zn (no3)2 + h2o. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: Oxidation number calculator is a free online tool that displays the oxidation number of. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT 7.3 Balancing Redox Reactions Using Oxidation Numbers What Is The Oxidation Number Of N In Zn No3 2 The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. The balanced equation will be calculated along with the oxidation. Enter an equation of a redox chemical reaction and press the balance button. Zn + 2 hno3 → zn (no3)2 + h2. Oxidation number. What Is The Oxidation Number Of N In Zn No3 2.
From byjus.com
For the redox reactionZn + NOg → Zn2+ +NH4+ in basic medium What Is The Oxidation Number Of N In Zn No3 2 Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. The balanced equation will be calculated along with the oxidation. Enter an equation of a redox chemical reaction and press the balance button. Zn + 2 hno3 → zn (no3)2 + h2. Enter the formula of a chemical compound to find the. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn No3 2 The balanced equation will be calculated along with the oxidation. Enter an equation of a redox chemical reaction and press the balance button. A net ionic charge can be specified at the end of the. Zno + 2 hno3 → zn (no3)2 + h2o. Zn + 2 hno3 → zn (no3)2 + h2. Enter the formula of a chemical compound. What Is The Oxidation Number Of N In Zn No3 2.
From www.toppr.com
Complete and balance the given reaction. Zn + NO3^ Zn^2 + + NH4^+ What Is The Oxidation Number Of N In Zn No3 2 The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. The balanced equation will be calculated along with the oxidation. Zn + 2 hno3 → zn (no3)2 + h2. A net ionic charge can be specified at the end of the. To calculate oxidation. What Is The Oxidation Number Of N In Zn No3 2.
From w20.b2m.cz
Zn Mg No3 2 EDUCA What Is The Oxidation Number Of N In Zn No3 2 The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of four valence electrons to the more. Enter an equation of a redox chemical reaction and press the balance button. Zn + 2 hno3 → zn (no3)2 + h2. These reactions are accompanied by the hydration of. Oxidation number calculator is. What Is The Oxidation Number Of N In Zn No3 2.
From shiken.ai
Oxidation Number What Is The Oxidation Number Of N In Zn No3 2 Enter the formula of a chemical compound to find the oxidation number of each element. The balanced equation will be calculated along with the oxidation. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for. What Is The Oxidation Number Of N In Zn No3 2.
From www.youtube.com
How to Balance Zn(NO3)2 = ZnO + NO2 + O2 (Zinc Nitrate What Is The Oxidation Number Of N In Zn No3 2 Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Enter an equation of a redox chemical reaction and press the balance button. Zno + 2 hno3 → zn (no3)2 + h2o. The nitrogen in the no 2 ion has an oxidation number of + 4, which corresponds to the loss of. What Is The Oxidation Number Of N In Zn No3 2.
From www.chemistrylearner.com
Oxidation Number (State) Definition, Rules, How to Find, and Examples What Is The Oxidation Number Of N In Zn No3 2 Zn + 2 hno3 → zn (no3)2 + h2. Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Zno + 2 hno3 → zn (no3)2 + h2o. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Enter the formula of a chemical. What Is The Oxidation Number Of N In Zn No3 2.
From studylib.net
Oxidation Numbers What Is The Oxidation Number Of N In Zn No3 2 Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical compound. Zn + 2 hno3 → zn (no3)2 + h2. Zno + 2 hno3 → zn (no3)2 + h2o. The balanced equation will be calculated along with the oxidation. The nitrogen in the no 2 ion has an oxidation number of + 4,. What Is The Oxidation Number Of N In Zn No3 2.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor What Is The Oxidation Number Of N In Zn No3 2 Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: These reactions are accompanied by the hydration of. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Oxidation number calculator is a free online tool that displays the oxidation number of the given chemical. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn No3 2 These reactions are accompanied by the hydration of. Enter the formula of a chemical compound to find the oxidation number of each element. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: Zno + 2 hno3 → zn (no3)2 + h2o. The balanced equation will be calculated along with the oxidation. To. What Is The Oxidation Number Of N In Zn No3 2.
From www.numerade.com
SOLVEDList the different nitrogen oxides. What is the oxidation number What Is The Oxidation Number Of N In Zn No3 2 A net ionic charge can be specified at the end of the. These reactions are accompanied by the hydration of. Zno + 2 hno3 → zn (no3)2 + h2o. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Zn + 2 hno3 → zn (no3)2 + h2. The nitrogen in the. What Is The Oxidation Number Of N In Zn No3 2.
From www.onlinechemistrytutor.net
Oxidation state examples Online Chemistry Tutor What Is The Oxidation Number Of N In Zn No3 2 Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: These reactions are accompanied by the hydration of. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for example: Zn + 2 hno3 → zn (no3)2 + h2. The nitrogen in the no 2 ion has. What Is The Oxidation Number Of N In Zn No3 2.
From www.slideserve.com
PPT Oxidation Numbers PowerPoint Presentation, free download ID5075015 What Is The Oxidation Number Of N In Zn No3 2 These reactions are accompanied by the hydration of. Zn + 2 hno3 → zn (no3)2 + h2. Zinc nitrate is usually prepared by dissolving zinc metal, zinc oxide, or related materials in nitric acid: Zno + 2 hno3 → zn (no3)2 + h2o. To calculate oxidation numbers of elements in the chemical compound, enter it's formula and click 'calculate' (for. What Is The Oxidation Number Of N In Zn No3 2.