Bromine Halogenation Mechanism . If you have a gaseous alkene like ethene, you can bubble it through either pure. Predict the two products of the allylic. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. The reaction with bromine happens at room temperature. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Write out the complete mechanism including reactants, intermediates and products. Cyclooctene undergoes radical allylic bromination.
from www.slideserve.com
Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Predict the two products of the allylic. Cyclooctene undergoes radical allylic bromination. Write out the complete mechanism including reactants, intermediates and products. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. The reaction with bromine happens at room temperature.
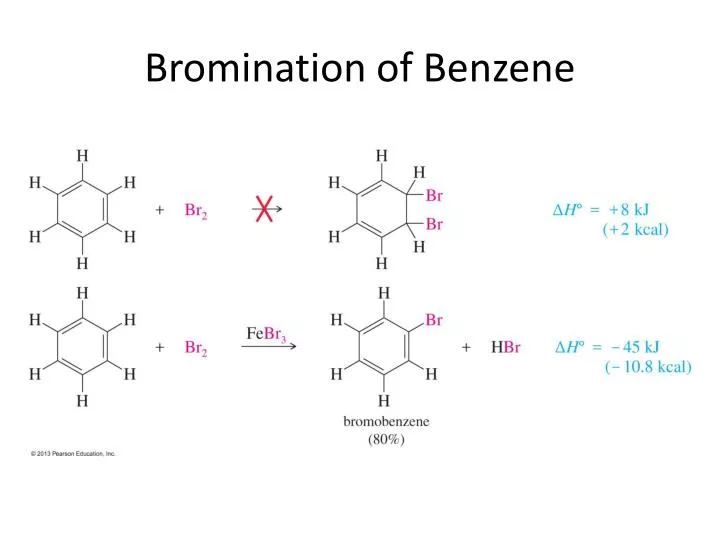
PPT Bromination of Benzene PowerPoint Presentation, free download
Bromine Halogenation Mechanism Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The reaction with bromine happens at room temperature. Cyclooctene undergoes radical allylic bromination. Write out the complete mechanism including reactants, intermediates and products. Predict the two products of the allylic. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution.
From www.masterorganicchemistry.com
Halogenation Of Ketones via Enols Master Organic Chemistry Bromine Halogenation Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction with bromine happens at room temperature. Predict the two products of the allylic. If you have a gaseous alkene like ethene, you can bubble it through either pure. Write out the complete mechanism including reactants, intermediates and. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Bromination of alkenes with Br2 to give dibromides Master Organic Bromine Halogenation Mechanism Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. Predict the two products of the allylic. The reaction with bromine happens at room temperature. Cyclooctene undergoes radical allylic bromination. Chlorination, iodination, and bromination of benzene). Bromine Halogenation Mechanism.
From www.chemistrylearner.com
Halogenation Definition, Examples, and Mechanism Bromine Halogenation Mechanism Cyclooctene undergoes radical allylic bromination. Write out the complete mechanism including reactants, intermediates and products. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in. Bromine Halogenation Mechanism.
From www.youtube.com
NBS Bromination YouTube Bromine Halogenation Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. The reaction with bromine happens at room temperature. Predict the two products of the allylic. Write out the complete mechanism including reactants, intermediates and products. Cyclooctene undergoes radical allylic bromination. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine. Bromine Halogenation Mechanism.
From chemistnotes.com
Allylic Halogenation/Benzylic Bromination Chemistry Notes Bromine Halogenation Mechanism Write out the complete mechanism including reactants, intermediates and products. Cyclooctene undergoes radical allylic bromination. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. If you have a gaseous alkene like ethene, you can bubble it through either pure. Predict. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromine Halogenation Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. Chlorination, iodination, and bromination of. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
What is Allylic Bromination? Master Organic Chemistry Bromine Halogenation Mechanism The reaction with bromine happens at room temperature. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Predict the two products of the allylic. Chlorination, iodination, and bromination of. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromine Halogenation Mechanism Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure. Cyclooctene undergoes radical allylic bromination. The reaction with bromine happens at room temperature. Write. Bromine Halogenation Mechanism.
From www.science-revision.co.uk
Halogenation of benzene rings Bromine Halogenation Mechanism Predict the two products of the allylic. Write out the complete mechanism including reactants, intermediates and products. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. If you have a gaseous alkene like ethene, you can bubble it through either pure. The reaction with bromine happens at room temperature. Draw the structure of the product formed when a given. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Halogenation Of Ketones via Enols Master Organic Chemistry Bromine Halogenation Mechanism Write out the complete mechanism including reactants, intermediates and products. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction with bromine happens at room temperature. Chlorination, iodination,. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Bromination of Alkenes Master Organic Chemistry Bromine Halogenation Mechanism Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. Predict the two products of the allylic. Cyclooctene undergoes radical allylic bromination. Alkenes react in the cold with pure liquid bromine, or with a solution of. Bromine Halogenation Mechanism.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Bromine Halogenation Mechanism Cyclooctene undergoes radical allylic bromination. The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. If you have a gaseous alkene like ethene, you can bubble it through either pure. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Draw. Bromine Halogenation Mechanism.
From www.organicchemistrytutor.com
Halogenation of Ketones and Haloform Reaction — Organic Chemistry Tutor Bromine Halogenation Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Cyclooctene undergoes radical allylic bromination. Write out the complete mechanism including reactants, intermediates and products. Predict the two products of the allylic. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine. Bromine Halogenation Mechanism.
From byjus.com
Explain the reaction mechanism of addition of bromine on acetylene by Bromine Halogenation Mechanism Write out the complete mechanism including reactants, intermediates and products. If you have a gaseous alkene like ethene, you can bubble it through either pure. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. The reaction with bromine happens at room temperature. Predict the two products of the allylic. Cyclooctene undergoes radical allylic bromination. Alkenes react in the cold. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Bromine Halogenation Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. Write out the complete mechanism including reactants, intermediates and products. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Cyclooctene undergoes radical allylic bromination. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Bromine Halogenation Mechanism Write out the complete mechanism including reactants, intermediates and products. Predict the two products of the allylic. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Cyclooctene undergoes radical allylic bromination. The reaction with bromine happens at room temperature. Alkenes. Bromine Halogenation Mechanism.
From www.aquaportail.com
Halogénation définition illustrée et explications Bromine Halogenation Mechanism Write out the complete mechanism including reactants, intermediates and products. Cyclooctene undergoes radical allylic bromination. Predict the two products of the allylic. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The reaction with bromine happens at room temperature. Alkenes react in the cold with pure liquid bromine, or with. Bromine Halogenation Mechanism.
From www.chemistrysteps.com
Halogenation of Benzene Chemistry Steps Bromine Halogenation Mechanism Cyclooctene undergoes radical allylic bromination. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. The reaction with bromine happens. Bromine Halogenation Mechanism.
From www.youtube.com
Bromination of Aniline Electrophilic Aromatic Substitution Reactions Bromine Halogenation Mechanism Cyclooctene undergoes radical allylic bromination. The reaction with bromine happens at room temperature. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Write out the complete mechanism including reactants, intermediates and products. If you have a gaseous alkene like ethene,. Bromine Halogenation Mechanism.
From vvtimenu.weebly.com
Bromination mechanism chem draw vvtimenu Bromine Halogenation Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Cyclooctene undergoes radical allylic bromination. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. If you have a gaseous. Bromine Halogenation Mechanism.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Bromine Halogenation Mechanism The reaction with bromine happens at room temperature. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. Write out the complete mechanism including reactants, intermediates and products. Cyclooctene undergoes radical allylic bromination. Predict the two. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Halogenation Mechanism Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Cyclooctene undergoes radical allylic bromination. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Write out the complete mechanism. Bromine Halogenation Mechanism.
From leah4sci.com
Halogenation of Alkenes Organic Chemistry Reaction Mechanism MCAT Bromine Halogenation Mechanism Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Cyclooctene undergoes radical allylic bromination. Predict the two products of the allylic. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Write out the complete mechanism including reactants, intermediates and products. Alkenes react in the cold with pure liquid bromine,. Bromine Halogenation Mechanism.
From chemtube3d.com
BaseCatalysed Bromination of Ketones Summary Bromine Halogenation Mechanism Cyclooctene undergoes radical allylic bromination. Predict the two products of the allylic. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. If you have a gaseous alkene like ethene, you can bubble it through either pure. Write out. Bromine Halogenation Mechanism.
From www.sketchite.com
Phenol Bromine Halogenation Reaction Bromination Water Cs2 Gives Reacts Bromine Halogenation Mechanism Predict the two products of the allylic. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. If you have a gaseous alkene like ethene, you can bubble it through either pure. Cyclooctene undergoes radical allylic bromination. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Alkenes react in the. Bromine Halogenation Mechanism.
From alaynameowzavala.blogspot.com
Cyclohexane Reaction With Bromine Bromine Halogenation Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Write out the complete mechanism including reactants, intermediates and products. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Electrophilic Aromatic Substitutions Chlorination and Bromination Bromine Halogenation Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. The reaction with bromine happens. Bromine Halogenation Mechanism.
From byjus.com
Halogenation Definition, Halogenation Types, Reactions, Importance Bromine Halogenation Mechanism Cyclooctene undergoes radical allylic bromination. Predict the two products of the allylic. Write out the complete mechanism including reactants, intermediates and products. If you have a gaseous alkene like ethene, you can bubble it through either pure. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. The reaction. Bromine Halogenation Mechanism.
From www.chemistrysteps.com
Allylic Bromination by NBS with Practice Problems Chemistry Steps Bromine Halogenation Mechanism Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Predict the two products of the allylic. Write out the complete mechanism including reactants, intermediates and products. Draw the structure of the product formed when a given alkene undergoes. Bromine Halogenation Mechanism.
From www.slideserve.com
PPT Bromination of Benzene PowerPoint Presentation, free download Bromine Halogenation Mechanism If you have a gaseous alkene like ethene, you can bubble it through either pure. Write out the complete mechanism including reactants, intermediates and products. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. The reaction with bromine happens at room temperature. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Benzylic Bromination and Benzylic Oxidation Master Organic Chemistry Bromine Halogenation Mechanism Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. If you have a gaseous alkene like ethene, you can bubble it through either pure. Cyclooctene undergoes radical allylic bromination. Write out the complete mechanism including reactants, intermediates and products. Predict. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Synthesis (2) Reactions of Alkanes Master Organic Chemistry Bromine Halogenation Mechanism Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene like ethene, you can bubble it through either pure. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Cyclooctene undergoes radical allylic bromination.. Bromine Halogenation Mechanism.
From newbedev.com
Chemistry Bromination on the aromatic ring vs aliphatic chain Bromine Halogenation Mechanism The reaction with bromine happens at room temperature. Write out the complete mechanism including reactants, intermediates and products. Predict the two products of the allylic. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. If you have a gaseous alkene. Bromine Halogenation Mechanism.
From study.com
Bromination of Acetanilide Mechanism & Steps Lesson Bromine Halogenation Mechanism The reaction with bromine happens at room temperature. Cyclooctene undergoes radical allylic bromination. Predict the two products of the allylic. Write out the complete mechanism including reactants, intermediates and products. Chlorination, iodination, and bromination of benzene) via electrophilic aromatic substitution. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like. Bromine Halogenation Mechanism.
From www.masterorganicchemistry.com
Bromination of Alkenes The Mechanism Master Organic Chemistry Bromine Halogenation Mechanism Cyclooctene undergoes radical allylic bromination. Write out the complete mechanism including reactants, intermediates and products. Draw the structure of the product formed when a given alkene undergoes an addition reaction with chlorine or bromine. Alkenes react in the cold with pure liquid bromine, or with a solution of bromine in an organic solvent like tetrachloromethane. Predict the two products of. Bromine Halogenation Mechanism.