Dilution Technique Measurement . Use the spectrophotometer to measure the absorbance of solutions. Generate a standard curve and use. The method involves four key functions: The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. The same can be said of a ratio. Convert to grams, or µg, uniformly. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. Injection of dye into the system. We are often concerned with how much solute is dissolved in a given amount of. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Understand how stock solutions are used in the laboratory. How to dilute solutions accurately and safely. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. Preparation of a “standard” of. State whether the concentration of a solution is directly or indirectly proportional to its volume.
from www.semanticscholar.org
We are often concerned with how much solute is dissolved in a given amount of. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. How to dilute solutions accurately and safely. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Generate a standard curve and use. State whether the concentration of a solution is directly or indirectly proportional to its volume. Preparation of a “standard” of. The same can be said of a ratio. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also.
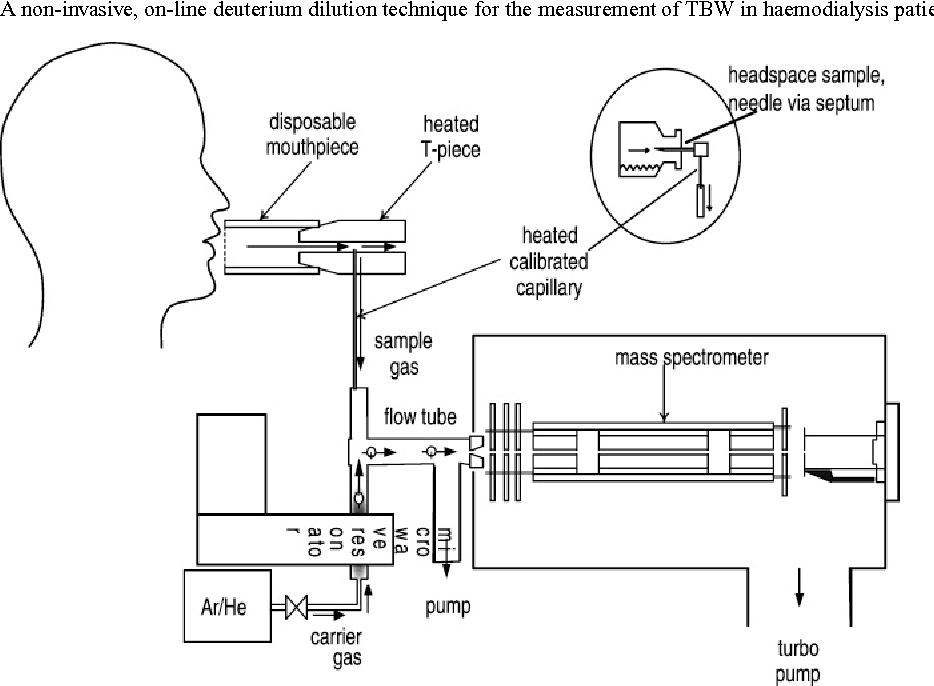
Figure 1 from A noninvasive, online deuterium dilution technique for the measurement of total
Dilution Technique Measurement The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Generate a standard curve and use. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. The method involves four key functions: Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. Understand how stock solutions are used in the laboratory. How to dilute solutions accurately and safely. We are often concerned with how much solute is dissolved in a given amount of. Use the spectrophotometer to measure the absorbance of solutions. Convert to grams, or µg, uniformly. Preparation of a “standard” of. Injection of dye into the system. The same can be said of a ratio. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution.
From chem.libretexts.org
5.2 Solutions and Dilutions Chemistry LibreTexts Dilution Technique Measurement Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. State whether the concentration of a solution is directly or indirectly proportional to its volume. Injection of dye into the system. Generate a standard curve and use. The same can be said of a ratio. A dilution is made. Dilution Technique Measurement.
From www.researchgate.net
Agar dilution method with NPs. Download Scientific Diagram Dilution Technique Measurement Injection of dye into the system. State whether the concentration of a solution is directly or indirectly proportional to its volume. The same can be said of a ratio. Preparation of a “standard” of. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. The method involves four key functions: Use the spectrophotometer. Dilution Technique Measurement.
From bio.libretexts.org
15 Determination of Bacterial Numbers Biology LibreTexts Dilution Technique Measurement Generate a standard curve and use. Use the spectrophotometer to measure the absorbance of solutions. Injection of dye into the system. We are often concerned with how much solute is dissolved in a given amount of. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also.. Dilution Technique Measurement.
From www.scribd.com
Stream Flow Measurement Dilution Technique PDF Flow Measurement Physical Quantities Dilution Technique Measurement Preparation of a “standard” of. Injection of dye into the system. We are often concerned with how much solute is dissolved in a given amount of. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. How to dilute solutions accurately and safely. The method involves four key functions:. Dilution Technique Measurement.
From dokumen.tips
(PDF) Discharge measurement with salt dilution method in hessd DOKUMEN.TIPS Dilution Technique Measurement Injection of dye into the system. The method involves four key functions: We are often concerned with how much solute is dissolved in a given amount of. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. A dilution is made by combining a certain volume. Dilution Technique Measurement.
From www.slideserve.com
PPT Salt Dilution An Alternative Stream Discharge Measurement Technique PowerPoint Dilution Technique Measurement Generate a standard curve and use. Use the spectrophotometer to measure the absorbance of solutions. State whether the concentration of a solution is directly or indirectly proportional to its volume. The same can be said of a ratio. Preparation of a “standard” of. We are often concerned with how much solute is dissolved in a given amount of. Understand how. Dilution Technique Measurement.
From www.answersarena.com
[Solved] The dilution method with the suddeninjection pr Dilution Technique Measurement Use the spectrophotometer to measure the absorbance of solutions. Injection of dye into the system. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Preparation of a “standard” of. Generate a standard curve and use. The method involves four key functions: The same can be said of a ratio. Convert to grams,. Dilution Technique Measurement.
From doctorlib.info
How to Measure Blood Pressure, Blood Flow, and Cardiac Volumes Organization of the Dilution Technique Measurement Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Injection of dye into the system. Convert to grams, or µg, uniformly. Generate a standard curve and use. Use the spectrophotometer to. Dilution Technique Measurement.
From microbeonline.com
Minimum Inhibitory concentration (MIC) Broth dilution methodprocedure and interpretation Dilution Technique Measurement Use the spectrophotometer to measure the absorbance of solutions. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Preparation of a “standard” of. How to dilute solutions accurately and safely. Using a concentrate and diluting at the point of use is a great way to save on storage space and. Dilution Technique Measurement.
From www.carolina.com
Infographic—Lab Basics How to Perform Serial Dilutions Carolina Biological Supply Dilution Technique Measurement Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. How to dilute solutions accurately and safely. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Generate a standard curve and use. Injection of dye into. Dilution Technique Measurement.
From microbenotes.com
Serial Dilution Formula, Calculator, Method, Uses, Examples Dilution Technique Measurement The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. State whether the concentration of a solution is directly or indirectly proportional to its volume. Injection of dye into the system. Preparation of a “standard” of. How to dilute solutions accurately and safely. A dilution is made by combining a certain volume of. Dilution Technique Measurement.
From www.semanticscholar.org
Figure 2 from Flow Measurement Using the Dye Dilution Technique Semantic Scholar Dilution Technique Measurement Preparation of a “standard” of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Generate a standard curve and use. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. Use the spectrophotometer to measure the absorbance of solutions. Convert to grams, or. Dilution Technique Measurement.
From www.researchgate.net
(PDF) Comparison of the accuracy of the lithium dilution technique with the thermodilution Dilution Technique Measurement The same can be said of a ratio. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. Use the spectrophotometer to measure the absorbance of solutions. Using a concentrate and diluting. Dilution Technique Measurement.
From www.researchgate.net
(PDF) Measurement of discharge rate in a canal using radiotracer dilution technique Dilution Technique Measurement Injection of dye into the system. Convert to grams, or µg, uniformly. We are often concerned with how much solute is dissolved in a given amount of. Understand how stock solutions are used in the laboratory. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. State whether the concentration of a solution. Dilution Technique Measurement.
From www.slideserve.com
PPT Stream Flow Measurement/Monitoring PowerPoint Presentation, free download ID2168345 Dilution Technique Measurement The same can be said of a ratio. Generate a standard curve and use. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Convert to grams,. Dilution Technique Measurement.
From www.slideserve.com
PPT Salt Dilution An Alternative Stream Discharge Measurement Technique PowerPoint Dilution Technique Measurement How to dilute solutions accurately and safely. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than. Dilution Technique Measurement.
From www.researchgate.net
Schematic representation of the (a) isotope pool dilution experiment,... Download Scientific Dilution Technique Measurement Convert to grams, or µg, uniformly. We are often concerned with how much solute is dissolved in a given amount of. How to dilute solutions accurately and safely. Injection of dye into the system. The same can be said of a ratio. The method involves four key functions: A dilution is made by combining a certain volume of reagent or. Dilution Technique Measurement.
From derangedphysiology.com
LiDCO lithium dilution cardiac output measurement Deranged Physiology Dilution Technique Measurement Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. Understand how stock solutions are used in the laboratory. State whether the concentration of a solution is directly or indirectly proportional to its volume. Convert to grams, or µg, uniformly. Injection of dye into the system.. Dilution Technique Measurement.
From borenew.weebly.com
Serial Dilution Calculation Examples borenew Dilution Technique Measurement The method involves four key functions: Understand how stock solutions are used in the laboratory. Generate a standard curve and use. How to dilute solutions accurately and safely. Preparation of a “standard” of. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. Convert to grams, or µg, uniformly.. Dilution Technique Measurement.
From www.semanticscholar.org
Figure 1 from Use of a Local Indicator Dilution Technique for the Measurement of Oscillatory Dilution Technique Measurement We are often concerned with how much solute is dissolved in a given amount of. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Use the spectrophotometer to. Dilution Technique Measurement.
From www.slideserve.com
PPT Salt Dilution An Alternative Stream Discharge Measurement Technique PowerPoint Dilution Technique Measurement State whether the concentration of a solution is directly or indirectly proportional to its volume. We are often concerned with how much solute is dissolved in a given amount of. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Use the spectrophotometer to measure the absorbance of solutions. Convert to. Dilution Technique Measurement.
From www.pinterest.com
Dilution isolation technique Microbiology, Medical laboratory science, Microbiology lab Dilution Technique Measurement The method involves four key functions: State whether the concentration of a solution is directly or indirectly proportional to its volume. How to dilute solutions accurately and safely. Generate a standard curve and use. Understand how stock solutions are used in the laboratory. Preparation of a “standard” of. A dilution is made by combining a certain volume of reagent or. Dilution Technique Measurement.
From www.youtube.com
Serial Dilution Technique For Microbiological & Chemical Analysis Method, Example Dilution Technique Measurement Convert to grams, or µg, uniformly. Preparation of a “standard” of. The method involves four key functions: How to dilute solutions accurately and safely. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Injection of dye into the system. Generate a standard curve and use. Helium dilution •it is a method recommended. Dilution Technique Measurement.
From www.researchgate.net
25. Salt dilution method for measurement of local film flow rate... Download Scientific Diagram Dilution Technique Measurement Understand how stock solutions are used in the laboratory. The method involves four key functions: How to dilute solutions accurately and safely. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. We are often concerned with how much solute is dissolved in a given amount of. Convert to grams, or µg, uniformly.. Dilution Technique Measurement.
From www.youtube.com
Serial Dilution Method Protocol Step Wise Explanation YouTube Dilution Technique Measurement Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. Understand how stock solutions are used in the laboratory. Generate a standard curve and use.. Dilution Technique Measurement.
From www.shutterstock.com
Indicator Dilution Method Measuring Fluid Volumes Stock Vector (Royalty Free) 1763243204 Dilution Technique Measurement Injection of dye into the system. Convert to grams, or µg, uniformly. Generate a standard curve and use. The same can be said of a ratio. Use the spectrophotometer to measure the absorbance of solutions. We are often concerned with how much solute is dissolved in a given amount of. How to dilute solutions accurately and safely. Preparation of a. Dilution Technique Measurement.
From www.youtube.com
Discharge measurement methods in stream Current meter method Flotation methodSalt Dilution Dilution Technique Measurement Generate a standard curve and use. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Use the spectrophotometer to measure the absorbance of solutions. We are often concerned with how much. Dilution Technique Measurement.
From www.semanticscholar.org
Figure 3 from A noninvasive, online deuterium dilution technique for the measurement of total Dilution Technique Measurement The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Preparation of a “standard” of. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. Convert to grams, or µg, uniformly. How to dilute solutions accurately and safely. A. Dilution Technique Measurement.
From www.youtube.com
How to Perform Serial Dilution for Bacterial Growth Measurement StepbyStep Guide YouTube Dilution Technique Measurement The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Using a concentrate and diluting at the point of use is a great way to save on storage space and transport costs, and also. Injection of dye into the system. Use the spectrophotometer to measure the absorbance of solutions. Convert to grams, or. Dilution Technique Measurement.
From www.semanticscholar.org
Figure 1 from A noninvasive, online deuterium dilution technique for the measurement of total Dilution Technique Measurement We are often concerned with how much solute is dissolved in a given amount of. State whether the concentration of a solution is directly or indirectly proportional to its volume. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. The same can be said of a ratio. The. Dilution Technique Measurement.
From derangedphysiology.com
Measurement of cardiac output by indicator dilution Deranged Physiology Dilution Technique Measurement The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. Understand how stock solutions are used in the laboratory. State whether the concentration of a solution is directly or indirectly proportional to its volume. The same can be said of a ratio. How to dilute solutions accurately and safely. Preparation of a “standard”. Dilution Technique Measurement.
From www.slideserve.com
PPT Biochemical Oxygen Demand (BOD) PowerPoint Presentation, free download ID6680553 Dilution Technique Measurement Preparation of a “standard” of. How to dilute solutions accurately and safely. State whether the concentration of a solution is directly or indirectly proportional to its volume. Generate a standard curve and use. Use the spectrophotometer to measure the absorbance of solutions. The method involves four key functions: A dilution is made by combining a certain volume of reagent or. Dilution Technique Measurement.
From microbeonline.com
Serial Dilution Method for Estimating Viable Count of Bacteria • Microbe Online Dilution Technique Measurement Use the spectrophotometer to measure the absorbance of solutions. Helium dilution •it is a method recommended for routine measurement of lung volumes in patients other than those with communicable diseases. Preparation of a “standard” of. The method involves four key functions: How to dilute solutions accurately and safely. Generate a standard curve and use. The same can be said of. Dilution Technique Measurement.
From lasopatank436.weebly.com
Serial vs parallel dilution method microbiology lasopatank Dilution Technique Measurement The same can be said of a ratio. Injection of dye into the system. Preparation of a “standard” of. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. Convert to grams, or µg, uniformly. The method involves four key functions: Understand how stock solutions are used in the laboratory. The. Dilution Technique Measurement.
From journals.physiology.org
Fluorescence dilution technique for measurement of albumin reflection coefficient in isolated Dilution Technique Measurement Injection of dye into the system. Understand how stock solutions are used in the laboratory. A dilution is made by combining a certain volume of reagent or specimen with a certain volume of diluent. The method involves four key functions: The difference is a dilution is expressed as parts reagent or specimen to total parts of solution. State whether the. Dilution Technique Measurement.